POSS-based hybrid cationic copolymers with low aggregation potential for efficient gene delivery†
Shan Jiangab,
Ying Zhou Poha and
Xian Jun Loh*acd
aInstitute of Materials Research and Engineering, A*STAR (Agency for Science, Technology and Research), 3 Research Link, Singapore 117602
bCollege of Chemistry, Jilin University, Changchun, P. R. China 130012
cDepartment of Materials Science and Engineering, National University of Singapore, 9 Engineering Drive 1, Singapore 117576
dSingapore Eye Research Institute, 11 Third Hospital Avenue, Singapore 168751. E-mail: lohxj@imre.a-star.edu.sg
First published on 17th August 2015
Abstract
In this study, the synthesis and gene transfection efficiency of a series of novel hybrid amphiphilic copolymers consisting of dimethylaminoethyl methacrylate (DMAEMA), polyhedral oligomeric silsesquioxane (POSS), poly(ethylene glycol) methacrylate (PEGMA), and glycidyl methacrylate (GMA) monomeric segments are reported. The copolymers were synthesized by the atom transfer radical polymerization (ATRP) method. The composition and structural information of the synthesized copolymers were characterized by gel permeation chromatography (GPC) and 1H nuclear magnetic resonance spectroscopy (NMR). The copolymer can successfully condensed the plasmid DNA (pDNA) into nanoparticles. The cytotoxicity studies were performed using MTT assay and the copolymers exhibited relatively low cytotoxicity. The incorporation of PEGMA reduced the aggregation of the polyplexes in serum containing medium. The main objective of this project was achieved as it was demonstrated that polyplexes derived from the synthesized copolymers exhibited superior gene transfection efficiency in human embryonic kidney 293T (HEK 293T) cells than the control homopolymer.
Introduction
Gene therapy is the therapeutic delivery of genetic material into cells to modify or supplement genes for treatment of cancers and genetic diseases. Recently, the successful delivery of genetic material into cells has been the topic of intense research. The delivery of DNA encodes and expresses therapeutic proteins resulting in gene silencing or gene expression, to correct genetic mutations. Gene delivery can be achieved by microinjection of plasmid DNA, cell electroporation and the use of gene delivery carriers, either viral vectors or synthetic vectors.1–3 Synthetic vectors are favoured over viral vectors as the synthetic vectors possess better biocompatibility, lower immunogenicity, of lower cost and the production can be easily scaled up.3–6 Today, researchers face the challenge of designing gene delivery vectors with low cytotoxicity and high gene transfection efficiency.7 Over the years, cationic polymers for example, polyethyleneimine (PEI), chitosan and poly(dimethylaminoethyl methacrylate) (PDMAEMA) have been produced for gene delivery.7–11PDMAEMA has gained considerable attention among researchers recently due to its easy modification via copolymerization techniques and availability of amine moieties for complexation, making it ideal as a synthetic gene delivery vector.7,12 PDMAEMA is also a temperature- and pH-responsive hydrophilic polymer, rendering it useful in dual-responsive chemotherapeutic drug carrier systems due to its ability to remain stable at physiological pH 7 but can be stimuli-triggered to release encapsulated drug in vicinity of acidic extracellular environment of cancer cells at low pH of 5–6.5.7 A design strategy was implemented with DMAEMA as the first monomeric candidate of the novel synthetic gene delivery agent. Polyhedral oligomeric silsesquioxane (POSS) was then chosen as the hydrophobic and inorganic constituent of the copolymer based on three motivations. Firstly, the hydrophobicity of POSS allows for hydrophobic chemo-drug encapsulation via hydrophobic interactions. Micelles possessing hydrophobic groups have been previously studied by our group in terms of dye encapsulation.13–16 We believe that POSS will also induce a similar effect. Secondly, POSS has been shown to improve the biocompatibility in nano-composite materials. Lastly, POSS is able to disperse the cationic charges and thus facilitating gene transfection by lowering the cytotoxicity.17 Next, inclusion of poly(ethylene glycol) methacrylate (PEGMA) has been demonstrated to improve gene transfection efficiency, reduce cytotoxicity and most importantly, to improve water solubility and prevent aggregation of the nanoparticles.18–20 The selection of the final monomer of the copolymer boils down to the novelty and unique functionalities that can be explored and exploited. Glycidyl methacrylate (GMA) is a novel hydrophobic component of the copolymer that can be functionalized via ring-opening reactions (i.e. conjugate targeting amine moieties) to bind with anionic DNA for efficient gene delivery.8,21
This work reports the synthesis of novel hybrid cationic copolymers with both organic components (i.e. DMAEMA, PEGMA and GMA) and inorganic POSS via atom transfer radical polymerization (ATRP) to for effective gene delivery. Hybrid materials refer to the presence of both organic and inorganic components in the composites.22 The cationic copolymer allows complexation with anionic pDNA to produce nano-sized polyplexes by electrostatic interactions.7,23 The polyplexes derived from the synthesized copolymers demonstrated a low cytotoxicity and good gene transfection efficiency in human embryonic kidney 293T (HEK293T) cells than the homopolymer. These novel copolymers display significant potential as effective drug-encapsulated gene delivery agents for future gene therapy.
Experimental
Materials
Ethyl 2-bromoisobutyrate (EBiB), 1,1,4,7,10,10-hexamethyltriethylenetetramine (HMTETA), copper(I)bromide (CuBr), 2-propanol (isopropanol/IPA), dimethylaminoethylmethacrylate (DMAEMA), methacrylisobutyl polyhedral oligomeric silsesquioxane (POSS-MA), and poly(ethylene glycol) methacrylate (PEGMA) were purchased from Sigma-Aldrich and used as received, with the exception of glycidyl methacrylate (GMA) that was subsequently treated with inhibitor removal through column filtration before use.Synthesis of poly(POSS-r-GMA-r-PEGMA-r-DMAEMA) (POGED) hybrid copolymers by ATRP
A series of poly(POSS-r-GMA-r-PEGMA-r-DMAEMA) hybrid copolymers were synthesized from POSS-MA, GMA, PEGMA and DMAEMA monomers via ATRP. The copolymerization was random, as denoted by r. The synthesized copolymers are denoted as POGED, where the first P stands for poly-, the O represents POSS, G for GMA, E for PEGMA and D represents DMAEMA. As control, linear PDMAEMA was synthesized. POGED copolymers were synthesized via ATRP with molar feed ratios of POSS–DMAEMA fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115 and varying feed ratios of PEGMA and GMA.
115 and varying feed ratios of PEGMA and GMA.
The ATRP reaction was performed in a 30 mL glass vial equipped with a magnetic stirrer under the typical conditions of ATRP. As an example, the synthesis of copolymer POGED-1 is described. DMAEMA (4.00 g, 25.4 mmol), POSS-MA (0.20 g, 0.22 mmol), GMA (1.00 g, 7.04 mmol), PEGMA (1.00 g, 2.78 mmol), EBiB (0.08 g, 0.40 mmol), and HMTETA (0.09 g, 0.40 mmol) were added into the vial containing 10 mL of isopropanol. After the reactants had completely dissolved, the reaction mixture was bubbled with nitrogen for 30 min to remove oxygen. Then, trace amounts of copper(I)bromide catalyst was introduced into the vial under a nitrogen atmosphere. The reaction mixture was purged with nitrogen for an additional 10 min and the vial was then sealed under a nitrogen atmosphere. Under continuous stirring of 300 rpm, the reaction was allowed to proceed at 50 °C for 24 h. The polymerization was halted by dilution of the polymer solution with tetrahydrofuran (THF). Subsequently, the copper catalyst complex was eliminated by running the polymer solution through a basic aluminum oxide column. THF was removed with a rotary evaporator, producing a concentrated polymer solution in a round bottom flask. The crude products were precipitated in excess hexane and centrifuged for 5 min to remove the unreacted monomers. The copolymers obtained were dried in vacuum overnight at 40 °C. The copolymers were then re-dissolved in distilled water, centrifuged and freeze-dried to further purify the copolymers by removing the unreacted hydrophobic POSS-MA monomer as well as remaining impurities.
Preparation of the polymer/pDNA complexes
Plasmid DNA (pDNA) was amplified in E. coli bacteria and purified by EndoFree Plasmid Mega Kit (Qiagen). All the copolymer stock solutions were prepared with the same N% concentration of 10 mM. 50 μL of pDNA solution at 100 μg mL−1 was mixed in the copolymer stock solutions with N/P ratio from 0 to 4. Distilled water was added to ensure all samples have a fixed volume of 100 μL. Each sample was vortexed and incubated for approximately 30 min at room temperature.Gel retardation experiments
1 g of agarose powder was dissolved in 100 mL of Tris–acetate–EDTA (TAE) buffer solution. The solution was heated to dissolve the agarose completely. Then, 10 μL of GelRed™ fluorescent DNA stains (Biotium) was added in agarose solution. The solution was poured into a tray and allowed to dry and form the 1% agarose gel. Gel electrophoresis experiment was performed in TAE running buffer with 20 μL of copolymer solution and 4 μL of loading dye in each well, at 100 V for 45 min in a Mini-Sub Cell system (Bio-rad Laboratories). DNA bands were visualized and photographed via a UV transilluminator and BioDoc-It imaging system.Cytotoxicity evaluations
Evaluation of the cytotoxicity of POGED was performed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in human embryonic kidney 293T (HEK 293T) cells. Cells were cultivated in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (PS) at 37 °C and 5% CO2. The cells at densities of 1 × 104 cells per well were seeded in a 96-well microtiter plate. After incubation of 24 h, the used cell media were replaced with fresh cell medium containing known concentrations of polymers from 15.6 μg mL−1 to 500 μg mL−1, before further incubation of the cells for 24 h. Subsequently, 10 μL of MTT solution mixed in phosphate buffered saline (PBS, 5 mg mL−1) was introduced into each well containing fresh cell medium, attaining a final MTT concentration of 0.5 mg mL−1. After 4 h, the cell media along with the unreacted dye were eliminated via aspiration. 100 μL of dimethyl sulfoxide (DMSO) was added into each well to reveal the purple formazan crystals. Finally, a microplate reader was utilized to measure the absorbance at 570 nm wavelength. The cell viability (%) of each polymer relative to the control cells without POGED was determined.In vitro gene transfection efficiency
24-well plates were seeded with HEK 293T cells at a density of 5 × 104 cells per well 24 h before gene transfection. The polyplexes were prepared by pipetting the copolymers into DNA solutions with N/P ratio of 5 to 100, followed by vortexing and incubation for 30 min. The polyplexes were cultivated in two different medium including serum-supplemented and serum-free medium, and incubated with cells for 4 h. The medium were replaced with 500 μL of fresh serum-supplemented medium, and the cells were allowed to incubate for another 20 h, resulting in a total transfection time of 24 h. Next, the cells were washed with PBS and lysed in 100 μL of lysis buffer per well. The lysed cells were placed on a rocking platform with gentle shaking for 30 min for complete lysing cells. 100 μL of assay reagent (substrate and buffer) was added in 20 μL of lysis sample in 96-well white solid plate and the luciferase activity was measured on a luminometer (Tecan, Infinite M200) for 10 s of integration time. The relative light units (RLUs) were normalized against protein concentration in the cell samples, which was measured using a CommassiePlus™ assay kit (Pierce). The results were collected in triplicates and expressed as relative light units per milligram of cell protein lysate (RLU per mg protein).Characterization
Gel permeation chromatography (GPC) analysis was carried out with a Shimadzu SCL-10A and LC-8A system to determine the molecular weight (Mn, g mol−1) and polydispersity index (PDI) of the copolymers. Acetate buffer solution was used as the eluent for aqueous GPC analysis at a flow rate of 0.60 mL min−1 1H nuclear magnetic resonance spectroscopy (NMR) was utilized to confirm the molecular structure of the copolymers as well as to demonstrate that the copolymers were successfully synthesized. The 1H NMR (400 MHz) spectra were recorded on a Bruker AV-400 NMR spectrometer at room temperature using deuterated chloroform (CDCl3) as the solvent. Chemical shifts are reported in ppm with reference to the solvent peaks (δ = 7.3 ppm for CHCl3, δ = 4.7 ppm for HOD). Elemental analysis (EA) was performed using a FlashEA 1112 series CHNS–O analyzer via the dry combustion method to determine the total nitrogen content (%) of the copolymers for assessment of DNA-binding ability as well as gene transfection efficiency in the subsequent part of the experiment. Dynamic light scattering (DLS) was used to determine the hydrodynamic particle size of the polyplexes. The nanoplexes were pipetted into a 96-well microplate and polyplex size measurements were made on the DynaPro Plate Reader II (WYATT technology) equipped with a high sensitivity detecting laser and a microplate reader. Experiments were carried out in triplicate.Statistical analysis
Statistical analyses were conducted using SPSS 15.0. Data were expressed as mean ± SD of separate experiments in different transfection experiments. The medians between pairs of groups were compared using the student's t-test. A two-tailed p-value less than or equal to 0.05 with 95% confidence intervals was considered statistically significant. The conclusions were presented only when the outcome was statistically significant.Results and discussion
Synthesis of the POGED hybrid random copolymers by ATRP
ATRP was employed for the polymerization of POGED copolymers (Fig. 1) under standard ATRP conditions. POGED copolymers were synthesized in IPA solvent using EBiB as initiator at 50 °C and 300 rpm for 24 h. Three POGED copolymers (namely POGED-1, POGED-2, POGED-3) of varying monomer feed ratio were successfully synthesized. The linear PDMAEMA homopolymer was also synthesized to serve as the control of the experiment. 8 samples of poly(DMAEMA–POSS) were initially synthesized prior to the POGED copolymers to investigate their solubility in aqueous media as POSS is highly hydrophobic due to its bulky cage-like structure. At POSS-MA feed ratio greater than 5 wt%, the polymer solution formed a viscous and insoluble gel. The molar feed ratio of POSS-MA in POGED copolymers was thus adjusted to be lower than 5 wt% due to solubility considerations. PEGMA is reported to improve the biocompatibility of copolymers as well as to impart hydrophilicity, making the copolymers more water-soluble and reducing aggregation. It was explained that the good solvation properties of PEGMA due to its coordination of 2–3H2O molecules per ethylene oxide unit.24 Owing to the reduced water solubility of POSS, PEGMA was incorporated into POGED to reduce aggregation and more importantly, to confer water-solubility. Subsequent successful ATRP reactions produced a clear solution. To further purify them, the copolymers were dissolved in distilled water and centrifuge to remove the unreacted hydrophobic POSS-MA monomer as well as remaining impurities. The resulting copolymers were freeze-dried for following experiments.Polymer characterization
The molecular weights and PDI of POGED were determined from GPC and the results are summarized in Table 1. The molecular weight of the copolymer was designed to be around 1 × 104 g mol−1, to allow efficient excretion of the polymeric degradation products from the body through renal filtration.25 The molecular weights of the POGED copolymers are in good agreement with the design consideration, with their molecular weights close to 1 × 104 g mol−1. The GPC profiles are shown in Fig. S1†. The GPC traces displayed mono-modal profiles and shows that there were no unreacted monomers or oligomers in the sample. POSS-MA was removed by considering its aqueous insolubility compared to the water-soluble polymer. POGED-1, 2, 3 were produced with the increase of the weight ratio of DMAEMA units. Furthermore, the PDI of the copolymers were relatively low (1.45–1.47), as presented in Table 1, demonstrating a relatively uniform molecular weight distribution (MWD) of the POGED copolymers. The uniform MWD is attributed to the use of ATRP, which is a controlled radical polymerization that offers precise control of MW and MWD of the polymers.26 EA was used to determine the nitrogen content (%) in the copolymers (Table 1) for accurate assessment of their DNA-binding ability and gene transfection efficiency subsequently. It is crucial to note that the sole use of GPC is insufficient to justify the successful copolymerization of all four monomers namely: POSS, GMA, PEGMA and DMAEMA. This can be further confirmed using 1H NMR spectroscopy for precise molecular identification.| Copolymer | Weight ratioa (wt%) | Mn/g mol−1 (×103)b | PDIb | |||
|---|---|---|---|---|---|---|
| DMAEMA | POSS | PEGMA | GMA | |||
| a Weight ratio of four monomers and POSS was kept to be less than 5 wt%.b Determined by GPC. | ||||||
| POGED-1 | 64.5 | 3.23 | 16.1 | 16.1 | 9.99 | 1.47 |
| POGED-2 | 70.2 | 3.51 | 13.2 | 13.2 | 11.8 | 1.47 |
| POGED-3 | 76.9 | 3.85 | 9.62 | 9.62 | 11.7 | 1.45 |
| PDMAEMA | 100.0 | 0 | 0 | 0 | 9.93 | 1.21 |
1H NMR spectroscopy was utilized to characterize the molecular structure of the POGED copolymers. All proton signals belonging to PDMAEMA, POSS, GMA and PEGMA segments in POGED were confirmed. NMR spectra of all POGED copolymers displayed the same characteristic peaks, as the monomeric components are the same for all. The only difference lies in their peak intensities due to the difference in monomer feed ratios. As an example, the NMR spectrum of POGED-3, as shown in Fig. 2 is described. Characteristic peaks attributed to the PDMAEMA segments were observed at δ = 2.29 ppm ((N–CH3) of PDMAEMA), δ = 2.59 ppm ((N–CH3) of PDMAEMA) and δ = 4.05 ppm (–(CH2–O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) protons of PDMAEMA). For the POSS segment, the characteristic peak was observed at δ = 0.07– 0.60 ppm (methylene protons of POSS). The signals at δ = 3.55–3.64 ppm correspond to –CH2–CH2–O– of POSS and PEGMA. Signals observed at δ = 2.92 ppm and δ = 3.37 ppm are attributed to the epoxy protons of GMA. Lastly, protons in –CH3 and –CH2 of the poly(methacrylate) backbone in POGED copolymers were identified at δ = 0.88–1.05 ppm and δ = 1.81–2.0 ppm, respectively. To further confirm the absence of unreacted monomers in POGED, resonances observed at δ = 5.75 and δ = 6.2 ppm for vinyl protons of starting monomers were absent from the NMR spectra, indicating that the copolymerization of all components with high purity was successfully achieved.7,12,27
O) protons of PDMAEMA). For the POSS segment, the characteristic peak was observed at δ = 0.07– 0.60 ppm (methylene protons of POSS). The signals at δ = 3.55–3.64 ppm correspond to –CH2–CH2–O– of POSS and PEGMA. Signals observed at δ = 2.92 ppm and δ = 3.37 ppm are attributed to the epoxy protons of GMA. Lastly, protons in –CH3 and –CH2 of the poly(methacrylate) backbone in POGED copolymers were identified at δ = 0.88–1.05 ppm and δ = 1.81–2.0 ppm, respectively. To further confirm the absence of unreacted monomers in POGED, resonances observed at δ = 5.75 and δ = 6.2 ppm for vinyl protons of starting monomers were absent from the NMR spectra, indicating that the copolymerization of all components with high purity was successfully achieved.7,12,27
DNA binding properties of cationic POGED copolymers
Agarose gel electrophoresis and particle size measurements were utilized to evaluate the ability of the cationic POGED copolymers in condensing pDNA into nanoparticles. Firstly, the formation of POGED–pDNA polyplexes was studied via their electrophoresis mobility on the agarose gel at various ratios of amino-group (in DMAEMA segments of POGED) to phosphate-group (in pDNA), defined as the N/P ratio. Fig. 3 demonstrates the gel retardation results of cationic POGED-pDNA polyplexes with increasing N/P ratios from 0 to 4 in comparison with PDMAEMA homopolymer control. PDMAEMA control complexed pDNA at N/P ratio of 1. POGED-1, 2, 3 copolymers (N content is 1.17, 3.20 and 3.62%) complexed with pDNA effectively at N/P ratios of 2, 1.5 and 1, respectively. The pDNA condensation capability of POGED copolymer increased, with the increase of wt% of PDMAEMA unit and the increasing N%. The higher PEG and GMA content in POGED appeared to decrease the complexation ability of the copolymer. This is likely due to charge dilution provided by the non-ionic PEG and GMA segments. | ||
| Fig. 3 Electrophoretic mobility of pDNA in POGED/DNA polyplexes in comparison with PDMAEMA/DNA polyplexes at various N/P ratios. | ||
The particle size of POGED/DNA polyplexes were measured by DLS at various N/P ratios and the results were plotted in Fig. 4. The particle size of POGED/pDNA polyplexes decreased with increasing N/P ratio until a critical N/P value. After which, the particle size remained moderately constant as the N/P ratio continued to increase. The diameters of the polyplexes (POGED) were approximately 100–200 nm, while the diameter of PDMAEMA polyplexes was 150 nm. TEM micrographs of the spherical polyplexes showed a slightly smaller size than that determined by DLS, most possibly owing to the dehydration effect when the polyplexes were imaged using TEM (Fig. S2†). The size of the polyplexes has been reported as a crucial factor in determining the extent of DNA uptake by cells. Furthermore, it is shown that particles with diameters less than 200 nm are less susceptible to elimination by the reticuloendothelial system (RES).7 As such, the particle size of the POGED polyplexes was satisfactory. The zeta potential of the cationic polymers (POGED series)/pDNA complexes are compared to those of the PDMAEMA (25 kDa)/pDNA complexes at various N/P ratios in Fig. S3†. Zeta potential is an indicator of surface charges on the polymer/pDNA nanoparticles. A positively charged surface allows electrostatic interaction with anionic cell surfaces and facilitates cellular uptake. For the pDNA complexes of the POGED polymers and PDMAEMA, the net surface charge increases dramatically as the N/P ratio increases from 0.05 to 20, and stabilizes at N/P ratios of 10 and above. After reaching the weight ratio of 10, the zeta potentials of the pDNA complexes of all the three cationic polymers, POGED series, are strongly positive and vary within the narrow range of 20–26 mV, which will give rise to similar affinity for cell surfaces. In addition, at weight ratios of 10 and above, the excess cationic polymers do not exert any significant effect on the zeta potential of the complexes. PDMAEMA, on the other hand, shows a slightly more positive zeta potential value than the POGED polymers, which could affect its toxicity towards cells.
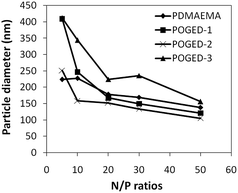 | ||
| Fig. 4 Particle size of the polyplexes formed from POGED copolymers and pDNA at various N/P ratios in comparison of pDMAEMA/pDNA nanoplex. | ||
Cytotoxicity of POGED copolymers
MTT is a colorimetric assay that examines the cytotoxicity of biomaterials via the selective ability of living cells with intact metabolic activity to reduce 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide into the purple formazan.23 Fig. 5 shows the in vitro cytotoxicity results of POGED copolymers as analyzed by the MTT assay in HEK 293T cells. The cell viability is inversely proportional to the cytotoxicity of the samples. Cationic polymers such as PDMAEMA have been reported to be damaging to cells due to permeabilization of cellular membranes, causing cell rupture.7 When PEGMA, POSS and GMA were incorporated into POGED-1 copolymer, the cell viability was slightly enhanced as compared to the PDMAEMA control at the similar molecular weight. The incorporation of PEGMA, POSS, and GMA allows for a reduction in cytotoxicity of the final copolymers. This phenomenon could be attributed to the dispersion of cationic groups, likely due to charge dilution, thereby reducing the cytotoxicity of the copolymers. Furthermore, POSS and PEGMA are known to be biocompatible and nontoxic which further explains the decrease in the cytotoxicity.7 When the incorporation amount of DMAEMA and POSS units increased, the resulting POGED-2 and 3 with higher molecular weight induced a lower cell viability compared with POGED-1. It was well-known that the cytotoxicity of polymer increases with the molecular weight.28 The estimated IC50 for POGED-1, 2 and 3 in HEK 293T cells were >500 μg mL−1, 384.1 μg mL−1, 264.9 μg mL−1 respectively. Clearly, it also shows that the lower zeta potential value of the POGED polymers compared with PDMAEMA also led to a lower toxicity profile of the polymers.In vitro gene transfection of POGED copolymers
In vitro gene transfection efficiency of POGED/pDNA polyplexes was assessed using luciferase as a marker gene in HEK 293T cells. Fig. 6 shows the gene transfection efficiency of POGED copolymers, as compared to PDMAEMA polymer, in presence and absence of serum. The transfection efficiency was greatly dependent on the N/P ratios, and it generally first increased at lower N/P ratios and then decreased with the increase in N/P ratios. At lower N/P ratios, pDNA cannot be condensed efficiently by the polymers, and the resultant loose polymer/pDNA complex cannot enter the cell easily. After reaching the optimal N/P ratios, the free cationic polymers in the polyplexes (besides the polymers that condense pDNA) cause increasing cytotoxicity, resulting in a reduction in the transfection efficiency. Fig. 6A shows that under complete serum conditions, the trend of gene transfection efficiency at optimal N/P ratios is as follows: POGED-1, POGED-2 > POGED-3 > PDMAEMA. The incorporation of PEGMA and POSS improves the gene transfection capability as compared with the linear PDMAEMA homopolymer. PEGMA reduces aggregation and facilitates the gene transfection.20 POSS is relatively hydrophobic and can help the formation of the polyplex nanoparticles by providing additional hydrophobic interaction.29,30 Furthermore, POGED-3 has lowest gene transfection efficiency among three POGED copolymers, indicating that the wt% of PDMAEMA unit in POGED copolymers should not be too high. Although higher PDMAEMA unit (i.e. POGED-3) amount gave better pDNA condensation capability (Fig. 3), its cytotoxicity also increased (Fig. 5), which is an important factor determining the transfection efficiency. At N/P ratio of 5, the transfection efficiency of POGED-3 was much higher than POGED-1 and 2, probably due to the higher DNA condensation ability. At N/P ratio of 5–20, the transfection efficiency of POGED-2 is higher than that of POGED-1, which may attribute to its relatively higher pDNA condensation ability (Fig. 3) and relatively smaller particle size (Fig. 4). In addition, under serum-free condition, although the transfection efficiency mediated by PDMAEMA homopolymer increased (in comparison with serum condition), which is similar to that of POGED-1 copolymers, POGED-1 copolymer remain the high transfection efficiency which is not affected by the serum presence. Overall, POGED-1 displayed the best gene transfection efficiency among all POGED copolymers and PDMAEMA homopolymer at optimum conditions. | ||
| Fig. 6 In vitro gene transfection efficiency of the cationic POGED/pDNA polyplexes in comparison to PDMAEMA control in HEK 293T cells in the (A) absence of serum (B) presence of serum. | ||
Conclusions
In this study, a series of novel hybrid amphiphilic copolymers (termed POGED) consisting of DMAEMA, POSS-MA, PEGMA and GMA monomers were successfully synthesized via the ATRP technique. The POGED copolymers were effectively characterized by GPC and 1H NMR. The results of the pDNA condensation ability of POGED via agarose gel electrophoresis and particle size measurements were optimistic. Owing to the incorporation of POSS and PEGMA, the POGED copolymers displayed better gene transfection efficiencies compared to the PDMAEMA homopolymer control. The findings in this study provide useful information for future designs of new POSS-based gene carriers.References
- Y. He, Y. Nie, G. Cheng, L. Xie, Y. Shen and Z. Gu, Adv. Mater., 2014, 26, 1632 CrossRef PubMed
.
- P. E. Boukany, Y. Wu, X. Zhao, K. J. Kwak, P. J. Glazer, K. Leong and L. J. Lee, Adv. Healthcare Mater., 2014, 3, 622 CrossRef PubMed
.
- M. Khan, Z. Y. Ong, N. Wiradharma, A. B. E. Attia and Y. Y. Yang, Adv. Healthcare Mater., 2012, 1, 373–392 CrossRef CAS PubMed
.
- Y. Yuan, C. J. Zhang and B. Liu, Angew. Chem., Int. Ed., 2015 DOI:10.1002/anie.201503640
.
- D. He and E. Wagner, Macromol. Biosci., 2015, 15, 600–612 CrossRef CAS PubMed
.
- X. Hu, H. Wang, J. Yang, W. Liu and W. Wang, J. Appl. Polym. Sci., 2014, 131 CAS
.
- X. J. Loh, S. J. Ong, Y. T. Tung and H. T. Choo, Mater. Sci. Eng., C, 2013, 33, 4545–4550 CrossRef CAS PubMed
.
- Y. Hu, Y. Zhu, W. Yang and F. Xu, ACS Appl. Mater. Interfaces, 2013, 5, 703–712 CAS
.
- S. Taranejoo, J. Liu, P. Verma and K. Hourigan, J. Appl. Polym. Sci., 2015, 132 Search PubMed
.
- J. W. Wang, C. Y. Chen and Y. M. Kuo, J. Appl. Polym. Sci., 2011, 121, 3531–3540 CrossRef CAS PubMed
.
- X. J. Loh, S. J. Ong, Y. T. Tung and H. T. Choo, Macromol. Biosci., 2013, 13, 1092–1099 CrossRef CAS PubMed
.
- Z. Li, D. Yuan, X. Fan, B. H. Tan and C. He, Langmuir, 2015, 31, 2321–2333 CrossRef CAS PubMed
.
- X. J. Loh, Z. X. Zhang, Y. L. Wu, T. S. Lee and J. Li, Macromolecules, 2009, 42, 194–202 CrossRef CAS
.
- X. J. Loh, J. S. Gong, M. Sakuragi, T. Kitajima, M. Z. Liu, J. Li and Y. Ito, Macromol. Biosci., 2009, 9, 1069–1079 CrossRef CAS PubMed
.
- X. J. Loh, W. C. D. Cheong, J. Li and Y. Ito, Soft Matter, 2009, 5, 2937–2946 RSC
.
- X. J. Loh, Y. L. Wu, W. T. J. Seow, M. N. I. Norimzan, Z. X. Zhang, F. Xu, E. T. Kang, K. G. Neoh and J. Li, Polymer, 2008, 49, 5084–5094 CrossRef CAS PubMed
.
- X. J. Loh, Z.-X. Zhang, K. Y. Mya, Y.-l. Wu, C. B. He and J. Li, J. Mater. Chem., 2010, 20, 10634–10642 RSC
.
- S. Venkataraman, W. L. Ong, Z. Y. Ong, S. C. J. Loo, P. L. R. Ee and Y. Y. Yang, Biomaterials, 2011, 32, 2369–2378 CrossRef CAS PubMed
.
- S. Lin, F. Du, Y. Wang, S. Ji, D. Liang, L. Yu and Z. Li, Biomacromolecules, 2007, 9, 109–115 CrossRef PubMed
.
- Y. Ikeda and Y. Nagasaki, J. Appl. Polym. Sci., 2014, 131 Search PubMed
.
- Y. Zhao, B. Yu, H. Hu, Y. Hu, N.-N. Zhao and F.-J. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 17911–17919 CAS
.
- M. Vallet-Regí, M. Colilla and B. González, Chem. Soc. Rev., 2011, 40, 596–607 RSC
.
- D. Fischer, Y. Li, B. Ahlemeyer, J. Krieglstein and T. Kissel, Biomaterials, 2003, 24, 1121–1131 CrossRef CAS
.
- G. Pasut, Polymers, 2014, 6, 160–178 CrossRef PubMed
.
- X. J. Loh, Z.-X. Zhang, Y.-L. Wu, T. S. Lee and J. Li, Macromolecules, 2008, 42, 194–202 CrossRef
.
- J. Wootthikanokkhan, M. Peesan and P. Phinyocheep, Eur. Polym. J., 2001, 37, 2063–2071 CrossRef CAS
.
- Z. Li, B. H. Tan, G. Jin, K. Li and C. He, Polym. Chem., 2014, 5, 6740–6753 RSC
.
- S. Xiang, H. Tong, Q. Shi, J. C. Fernandes, T. Jin, K. Dai and X. Zhang, J. Controlled Release, 2012, 158, 371–378 CrossRef CAS PubMed
.
- Y.-Y. Yang, X. Wang, Y. Hu, H. Hu, D.-C. Wu and F.-J. Xu, ACS Appl. Mater. Interfaces, 2013, 6, 1044–1052 Search PubMed
.
- F. Wang, X. Lu and C. He, J. Mater. Chem., 2011, 21, 2775–2782 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra12580d |
| This journal is © The Royal Society of Chemistry 2015 |



