Synthesis and in vitro antibacterial activity of novel fluoroalkyl-substituted pyrazolyl oxazolidinones†
Abstract
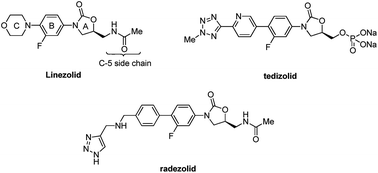
A series of novel oxazolidinone derivatives bearing fluoroalkyl-substituted pyrazole as the C-ring structure were designed, synthesized and evaluated for their antibacterial activity against six Gram-positive bacterial pathogens. Most of the target compounds have good antibacterial activity. Especially, compounds 13f, 13i and 13l show excellent activity comparable to linezolid.

 Please wait while we load your content...
Please wait while we load your content...