Palladium(II)-catalyzed Sequential ortho-olefination of β-arylethamines with assistance of oxalyl amide†
Pei Liu,
Jian Han,
Qian Wang,
Zhibin Huang,
Daqing Shi,
Runsheng Zeng* and
Ying Sheng Zhao*
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China. E-mail: yszhao@suda.edu.cn; zengrunsheng@suda.edu.cn
First published on 6th July 2015
Abstract
Pd-catalyzed highly selective ortho-alkenylation of β-arylethamines with the assistance of oxalyl amide is developed. A wide range of olefins such as allyl acetate, allyl alcohol derivatives, terminal alkenes, acraldehyde, acrylate, acrylonitrile, neohexene and styrene derivatives are all tolerable, which is the broadest yet reported for this kind of C–H transformation. Furthermore, sequential functionalization of β-arylethamine is also achieved well in one pot.
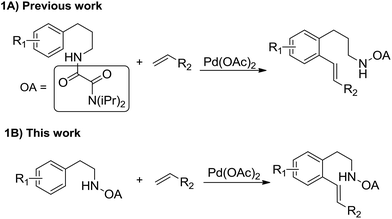
Transition-metal-catalyzed direct C–H functionalization reactions have emerged as an efficient methodology for constructing carbon–carbon and carbon–heteroatom bonds and has greatly enriched approaches to the synthesis of natural products and pharmaceutical agents in recent decades.1 Ever since the pioneering work by Fujiwara and Moritani on oxidative C–H olefination of arenes,2 transition-metal-catalyzed direct olefination of C–H bonds has been extensively explored.3–6 Recently, highly selective olefination with assistance of various directing groups, such as pyridine,7 amide,8 acid,9 hydroxyl10 and thioether11 has been achieved. For example, Leeuwen and co-workers developed a Pd-catalyzed oxidative coupling of anilides with olefins at room temperature in 2002.12 Later, the regioselective olefination of N,N-dimethylbenzylamines was developed by tuning the acidity of reaction conditions which was reported by Shi and co-workers.13 Alternatively, Chen and co-workers developed the γ-olefination of benzyl amine derivatives from vinyl halides under a limited substrates scope of olefins.14 However, the direct olefination of phenylethylamines at ortho-positions still has few reports. Yu and co-workers firstly disclosed a trifluoro-sulfonamide assisted mono-alkenylation of arylethamines around 50–65% yields with long reaction time.15 Very recently, the ortho-olefination of β-arylethamines was also developed by employing 1,2,3-triazole as the directing group via palladium as catalyst by Shi and co-workers with limited scope of olefins.16 Meanwhile, poor selectivity of mono- and di-olefination was observed. So far, sequential functionalization of β-arylethamines to construct complex olefinated arenes still has no reports. Herein, we reported a high selective palladium-catalyzed oxalyl amide-directed olefination of β-arylethamines with various olefins (Scheme 1B). Sequential ortho-olefination of β-arylethamines was also achieved in good yield in one pot.
Oxalyl amide has been discovered as an easily accessible and efficient directing group for amine derivatives by our group and employed in C–N, C–X, C–C bond formation via a seven or six-membered palladacycles17 (Scheme 1A). Encouraged by these oxalyl amide directed C–H transformations, we speculated that the sequential functionalization protected β-arylethamines could be achieved with oxalyl amide using palladium catalyst.
In a typical experiment, we first treated oxalyl amide protected phenylethylamine 1a, silver carbonate (2 equiv.) and allyl acetate with loading of 5 mol% of Pd(OAc)2 as catalyst in DCE at 80 °C under an atmosphere of air in a sealed tube. The product of 3a was observed in 50% yield detected by LC along with 42% starting material recovered (Table 1, entry 1). When dibutyl phosphate was used as the additive, 80% yield of 3a was obtained. Different oxidants, such as O2, Cu(OAc)2, BQ, Fe(OTf)3, and TEMPO were scanned, no one gave better yield than that of silver carbonate (entries 3–7). The application of PivOH which has been proved to have significant effects on many C–H activation reactions18 showed slightly promoting effect than that of silver carbonate in this transformation (entry 8). Both ortho-phenylbenzoic acid (o-PBA) and 1-AdOH had the similar effect in this reaction (entries 9–10). However, the amino acid (Ac-Gly-OH) and p-toluenesulfonamide (p-TSA) suppressed the reaction lightly compared to the pivalate acid, respectively (entries 11–12). A series of phosphoric acids were also screened (entries 13–14), and it turned out the dibutyl phosphate was the best additive in this synthetic protocol. Controlling experiments confirmed that no product was detected without palladium catalyst, implicating the crucial role of Pd(OAc)2 for the transformation. In addition, the dialkenylated product was also observed during the conditions screening. We reasoned that the palladium complex had a weak interaction with the olefin, which could block the dialkenylation reaction in a short reacting time (Table 2).
| Entrya | Oxidant | Additive | Yield (%) |
|---|---|---|---|
| a 1a (0.1 mmol), 2a (0.2 mmol), Pd(OAc)2 (5 mol%), oxidant (0.15 mmol), additive (0.03 mmol), DCE (0.3 mL) in a 15 mL sealed tube; yields were based on LC using acetophenone as the internal standard.b Isolated yield.c No catalyst was used. | |||
| 1 | Ag2CO3 | — | 50 |
| 2b | Ag2CO3 | (n-BuO)2PO2H | 80(71) |
| 3 | O2 | (n-BuO)2PO2H | 34 |
| 4 | Cu(OAc)2 | (n-BuO)2PO2H | 29 |
| 5 | BQ | (n-BuO)2PO2H | 35 |
| 6 | Fe(OTf)3 | (n-BuO)2PO2H | 20 |
| 7 | TEMPO | (n-BuO)2PO2H | <5 |
| 8 | Ag2CO3 | PivOH | 59 |
| 9 | Ag2CO3 | o-PBA | 62 |
| 10 | Ag2CO3 | 1-AdOH | 55 |
| 11 | Ag2CO3 | Ac-Gly-OH | 42 |
| 12 | Ag2CO3 | p-TSA | 46 |
| 13 | Ag2CO3 | (BnO)2PO2H | 75 |
| 14 | Ag2CO3 | Ph2PO2H | 69 |
| 15c | Ag2CO3 | (n-BuO)2PO2H | NR |
With the optimized conditions in hand, the scope of the oxalyl amide directed C–H ortho-olefination of phenylethylamines was examined (Table 2). Surprisingly, a remarkably broad substrate scope of amines was tolerable. Substrates bearing electron-donating and withdrawing groups such as –OMe, –CF3, –F were transformed into the corresponding alkenylated products in good to excellent yields (Table 2, 3b–3i). Notably, selective mono-alkenylation of substrates (3a–3c) were obtained in good yields along with less than 10% dialkenylated products respectively. The ortho-olefination selectively happened at the less hindered position in good yields (3e–3f, 3h). Furthermore, the oxalyl amide protected thiopheneethylamine (3g) gave the alkenylated products in synthetically useful yields. This new developed protocol was demonstrated good compatibility with various functionalized olefins, including hydroxyl, aldehyde, ketone, ether, cyano and styrene derivatives (Table 3). Gratifyingly, the terminal alkenes gave the product in good yields under standard conditions (3j–3u), along with around 10% starting material recovered respectively. The electron-deficient olefins such as acrylamide, acrylonitrile, acrylaldehyde, vinyl sulfone were more active than electron-rich olefins in the transformation, which resulted in more dialkenylated product respectively. It is worth to noting that a mixture of mono- and di-olefinated products was obtained when acrylate were used. When we increased the amount of acrylate, 90% yield of dialkenylated product 3v was achieved (Scheme 2).
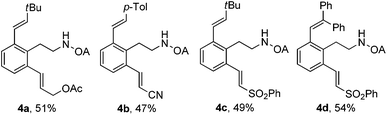
When oxalyl amide protected phenylethylamines reacted with electron-rich olefins, the reactions were clean, and only less than 5% dialkenylated products were observed, accompanied with starting material recovered. However, the electron-deficient olefins underwent high conversion of starting material and poorer selectivity of mono-, di-alkenylated products (Scheme 2). Thus, we might achieve the sequential oxalyl amide directed alkenylation reactions by combination of two different types of olefins. Satisfactorily, the sequential olefinations were successfully achieved in one pot, and the total yields were in synthetic acceptable yield (Table 4, 4a–4d).
| a 1 (0.5 mmol), 2 (1.0 mmol), Pd(OAc)2 (5 mol%), Ag2CO3 (0.75 mmol), (n-BuO)2PO2H (0.15 mmol), DCE (1.0 mL) at 80 °C, 18 h in a 15 mL sealed tube; then 2′ (1.0 mmol), Pd(OAc)2 (5 mol%), Ag2CO3 (0.75 mmol), (n-BuO)2PO2H (0.15 mmol); isolated yields. |
|---|
 |
To further demonstrate the utility of this protocol, the alkenylated products of 3k was arylated with 4-iodotoluene by using the general protocol reported by our group in good yield (Scheme 3).17c
In conclusion, we have developed a powerful protocol for palladium-catalyzed C(sp2)–H ortho-olefination of β-arylethylamines under the assistance of oxalyl amide. A wide range of olefins, such as terminal alkyl olefins, allyl alcohol derivative, acraldehyde, vinyl sulfone, and acrylonitrile were all successfully employed in this transformation. Impressively, sequential functionalization of β-arylethylamines was also observed which could be used in constructing the complex arenes. The detail mechanism study and exploring new applications for oxalyl amide assisted C–H transformation are now being undertaken in our laboratory.
Acknowledgements
We gratefully acknowledge financial support from the Natural Science Foundation of Jiangsu Province of China (BK20130294), and the Young National Natural Science Foundation of China (No. 21402133) for support of this work. The PAPD is also gratefully acknowledged.Notes and references
- For recent reviews: (a) K. Godula and D. Sames, Science, 2006, 312, 67–72 CrossRef CAS PubMed; (b) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147–1169 CrossRef CAS PubMed; (c) L. F. McMurray and M. J. Gaunt, Chem. Soc. Rev., 2011, 40, 1885–1898 RSC; (d) K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2011, 45, 788–802 CrossRef PubMed; (e) B.-J. Li and Z.-J. Shi, Chem. Soc. Rev., 2012, 41, 5588–5598 RSC; (f) J. J. Mousseau and A. B. Charette, Acc. Chem. Res., 2012, 46, 412–424 CrossRef PubMed; (g) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., 2012, 124, 9092–9142 (Angew. Chem., Int. Ed., 2012, 51, 8960–9009) CrossRef PubMed; (h) G. Rouquet and N. Chatani, Angew. Chem., 2013, 125, 11942–11959 (Angew. Chem., Int. Ed., 2013, 52, 11726–11743) CrossRef PubMed; (i) M.-L. Louillat and F. W. Patureau, Chem. Soc. Rev., 2014, 43, 901–910 RSC.
- (a) I. Moritani and Y. Fujiwara, Tetrahedron Lett., 1967, 1119–1123 CrossRef CAS; (b) Y. Fujiwara, I. Moritani, S. Danno, R. Asano and S. Teranishi, J. Am. Chem. Soc., 1969, 91, 7166–7170 CrossRef CAS.
- (a) C. Jia, T. Kitamura and Y. Fujiwara, Acc. Chem. Res., 2001, 34, 633–639 CrossRef CAS PubMed; (b) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068–5083 RSC; (c) P. Gandeepan, K. Parthasarathy and C.-H. Cheng, J. Am. Chem. Soc., 2010, 132, 8569–8671 CrossRef CAS PubMed; (d) E. M. Beck, N. P. Grimster, R. Hatley and M. J. Gaunt, J. Am. Chem. Soc., 2006, 128, 2528–2529 CrossRef CAS PubMed; (e) J. Karthikeyan and C.-H. Cheng, Angew. Chem., 2011, 123, 10054–10057 (Angew. Chem., Int. Ed., 2011, 50, 9880–9883) CrossRef PubMed; (f) D.-H. Wang, K. M. Engle, B.-F. Shi and J.-Q. Yu, Science, 2010, 327, 315–319 CrossRef CAS PubMed; (g) M. Ye, G.-L. Gao and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 6964–6967 CrossRef CAS PubMed.
- (a) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879–5918 CrossRef CAS PubMed; (b) L. Ackermann, A. V. Lygin and N. Hofmann, Angew. Chem., 2011, 123, 6503–6506 (Angew. Chem., Int. Ed., 2011, 50, 6379–6382) CrossRef PubMed; (c) L. Ackermann, L. Wang and A. V. Lygin, Chem. Sci., 2012, 3, 177–180 RSC; (d) V. Lanke and K. R. Prabhu, Org. Lett., 2013, 15, 2818–2821 CrossRef CAS PubMed; (e) R. Chidipudi, M. D. Wieczysty, I. Khan and H. W. Lam, Org. Lett., 2013, 15, 570–573 CrossRef PubMed.
- (a) D. A. Colby, A. S. Tsai, R. G. Bergman and J. A. Ellman, Acc. Chem. Res., 2012, 45, 814–825 CrossRef CAS PubMed; (b) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2009, 110, 624–655 CrossRef PubMed; (c) G. Song, F. Wang and X. Li, Chem. Soc. Rev., 2012, 41, 3651–3678 RSC; (d) T. Satoh and M. Miura, Chem. Eur. J., 2010, 16, 11212–11222 CrossRef CAS PubMed; (e) L. Ackermann, Chem. Rev., 2011, 111, 1315–1345 CrossRef CAS PubMed; (f) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068–5083 RSC.
- (a) Y.-H. Zhang, B.-F. Shi and J.-Q. Yu, J. Am. Chem. Soc., 2009, 131, 5072–5074 CrossRef CAS PubMed; (b) A. Kubota, M. H. Emmert and M. S. Sanford, Org. Lett., 2012, 14, 1760–1763 CrossRef CAS PubMed; (c) H. U. Vora, A. P. Silvestri, C. J. Engelin and J.-Q. Yu, Angew. Chem., 2014, 126, 2721–2724 (Angew. Chem., Int. Ed., 2014, 53, 2683–2686) CrossRef PubMed; (d) X. Zhang, S. Fan, C.-Y. He, X. Wan, Q.-Q. Min, J. Yang and Z.-X. Jiang, J. Am. Chem. Soc., 2010, 132, 4506–4507 CrossRef CAS PubMed.
- (a) A. García-Rubia, M. Á. Fernández-Ibáñez, R. G. Arrayás and J. C. Carretero, Chem. Eur. J., 2011, 17, 3567–3570 CrossRef PubMed; (b) A. García-Rubia, B. Urones, R. G. Arrayás and J. C. Carretero, Angew. Chem., 2011, 123, 11119–11123 (Angew. Chem., Int. Ed., 2011, 50, 10927–10931) CrossRef PubMed; (c) X. Cong, J. You, G. Gao and J. Lan, Chem. Commun., 2013, 49, 662–664 RSC.
- (a) F. Wang, G. Song and X. Li, Org. Lett., 2010, 12, 5430–5433 CrossRef CAS PubMed; (b) Q. Liu, Q. Li, Y. Ma and Y. Jia, Org. Lett., 2013, 15, 4528–4531 CrossRef CAS PubMed; (c) M. Wasa, K. M. Engle and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 3680–3681 CrossRef CAS PubMed.
- (a) K. M. Engle, D.-H. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 14137–14151 CrossRef CAS PubMed; (b) D.-H. Wang, K. M. Engle, B.-F. Shi and J.-Q. Yu, Science, 2010, 327, 315–319 CrossRef CAS PubMed.
- Y. Lu, D.-H. Wang, K. M. Engle and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 5916–5921 CrossRef CAS PubMed.
- (a) M. Yu, Y. Xie, C. Xie and Y. Zhang, Org. Lett., 2012, 14, 2164–2167 CrossRef CAS PubMed; (b) X.-S. Zhang, Q.-L. Zhu, Y.-F. Zhang, Y.-B. Li and Z.-J. Shi, Chem. Eur. J., 2013, 19, 11898–11903 CrossRef CAS PubMed.
- M. D. K. Boele, G. P. F. van Strijdonck, A. H. M. de Vries, P. C. J. Kamer, J. G. de Vries and P. W. N. M. van Leeuwen, J. Am. Chem. Soc., 2002, 124, 1586–1587 CrossRef CAS PubMed.
- G. Cai, Y. Fu, Y. Li, X. Wan and Z. Shi, J. Am. Chem. Soc., 2007, 129, 7666–7673 CrossRef CAS PubMed.
- Y. Zhao, G. He, W. A. Nack and G. Chen, Org. Lett., 2012, 14, 2948–2951 CrossRef CAS PubMed.
- J.-J. Li, T.-S. Mei and J.-Q. Yu, Angew. Chem., 2008, 120, 6552–6555 (Angew. Chem., Int. Ed., 2008, 47, 6452–6455) CrossRef PubMed.
- X. H. Ye and X. D. Shi, Org. Lett., 2014, 16, 4448–4451 CrossRef CAS PubMed.
- (a) C. Wang, C.-P. Chen, J.-Y. Zhang, Y.-M. Yao and Y.-S. Zhao, Angew. Chem., 2014, 126, 10042–10046 (Angew. Chem., Int. Ed., 2014, 53, 9884–9888) CrossRef PubMed; (b) Q. Wang, J. Han, C. Wang, J.-Y. Zhang, Z.-B. Huang, D.-Q. Shi and Y.-S. Zhao, Chem. Sci., 2014, 5, 4962–4967 RSC; (c) J. Han, P. Liu, C. Wang, Q. Wang, J.-Y. Zhang, Y.-W. Zhao, D.-Q. Shi, Z.-B. Huang and Y.-S. Zhao, Org. Lett., 2014, 16, 5682–5685 CrossRef CAS PubMed; (d) C.-P. Chen, C. Wang, J.-Y. Zhang and Y.-S. Zhao, J. Org. Chem., 2015, 80, 942–949 CrossRef CAS PubMed; (e) P. Liu, J. Han, C.-P. Chen, D.-Q. Shi and Y.-S. Zhao, RSC Adv., 2015, 5, 28430–28434 RSC.
- (a) M. Lafrance and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 16496–16497 CrossRef CAS PubMed; (b) D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2010, 132, 3965–3972 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra11677e |
| This journal is © The Royal Society of Chemistry 2015 |