Stereoisomerism metabolites found in rats after oral administration of timosaponin B-II using HPLC-Q-TOF-MS and NMR methods†
Zhiwen Fu‡
ab,
Zhixiong Li‡a,
Rui Xuea,
Jian Jiang*b and
Chenggang Huang*a
aShanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, P.R. China. E-mail: cghsimm@126.com
bShuguang Hospital affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 201203, P.R. China
First published on 6th July 2015
Abstract
Timosaponin B-II (TB-II), a representative furostanol saponin from Rhizoma Anemarrhenae, has been used to treat diabetes and senile dementia. To better understand the action mechanism of TB-II, it is indispensable to study its metabolism profile in vivo. A HPLC-Q-TOF-MS and NMR methods for the analysis of TB-II and its metabolites in rat urine has been developed. Five derivatives of TB-II including one novel compound (timosaponin BIII-d) were synthesized and used as the reference compounds. In rat urine, a total of 13 metabolites were identified after oral administration of TB-II and eight of them were confirmed by NMR. The metabolic pathways of TB-II were mainly deglycosylation, F-ring isomerization, and even stereoisomerism at C25. It is important for understanding metabolism of TB-II in rats to clarify its metabolic pathways in vivo, which will also provide useful information and reference for similar study in humans. Moreover, the metabolites could contribute to obtain better compounds than TB-II for the development of new drugs in the future.
Introduction
As an important component of many well-known traditional Chinese prescriptions, Rhizoma Anemarrhenae has been clinically employed for the treatment of diabetes and senile dementia for hundreds of years. Timosaponin B-II (TB-II), a natural bioactive steroid glycoside (Fig. 1), is one of the major active ingredients in Rhizoma Anemarrhenae. According to the recent pharmacological research, it has been reported that TB-II has neuronal protective and anti-inflammatory effects possibly by suppressing the production of pro-inflammatory factors IL-1, IL-6 and TNF-α.1–3 It could also improve the ability of learning and memory by promoting scavenging of superoxide radicals in the αβ-induced dementia models.4 Moreover, there is a possibility that it is regarded as a potential lead drug candidate used for senile dementia since TB-II could significantly ameliorate the learning and memorizing abilities in memory-deficit rat models.5Attention has been raised concerning the potential anti-dementia effects of TB-II. However, it was poorly absorbed via the gastrointestinal tract with the absolute oral bioavailability being only 1.1 ± 0.3%.6,7 Therefore, it is important to investigate the profile of metabolites of TB-II obtained after in vivo metabolisation to understand its action mechanism. As of today, there are only limited studies performed, and most metabolites were only predicted by their mass spectra information,8 which is not sufficient for structural identification without the use of NMR information.
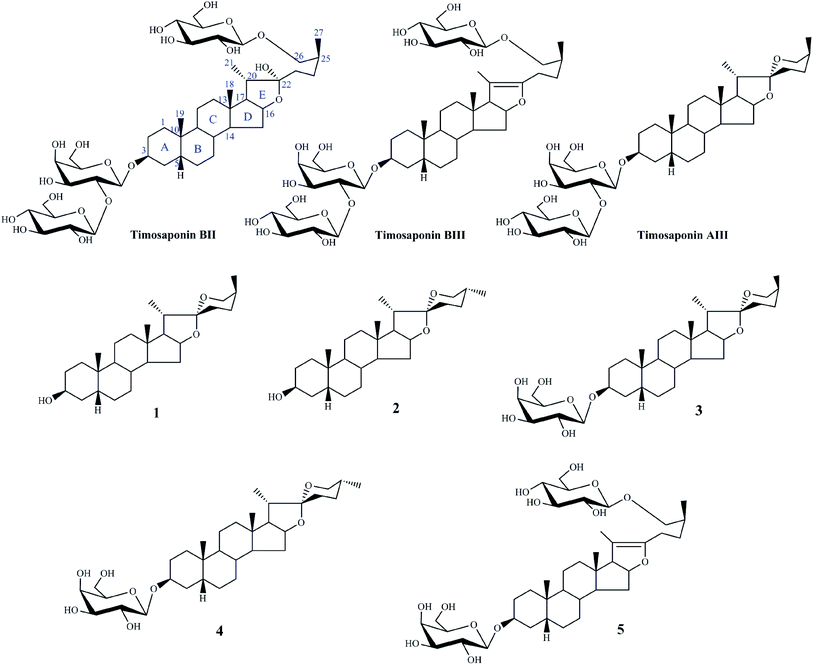
To take into account the advantages of NMR information for structure confirmation, this work explored the use of a practical approach to synthesize some derivatives of TB-II acting as the reference compounds for LC-MS analysis. According to the characteristics of the chemical structure of TB-II and the previous LC-MS analysis results,8 the major biotransformation pathway of TB-II in vivo was deglycosylation. Therefore, in this work, three timosaponin standards (TB-II, timosaponin B-III, and timosaponin A-III) as well as five deglycosylation derivates of TB-II including a novel one (timosaponin BIII-d) were obtained (Fig. 1, 1–5) and used as the reference compounds. Furthermore, a high performance liquid chromatography in combination with quadrupole-time-of-flight mass spectrometry (HPLC-Q-TOF-MS) method was established to rapidly investigate the metabolism in rat urine following oral administration of TB-II.
Materials & methods
Chemicals & reagents
Hydrazine acetate was purchased from Sigma-Aldrich (Beijing, China). 1,8-Diazabicyclo[5,4,0]-7-ene (DBU), trichloroacetonitrile, and trimethylsllytrifluoromethanesulphonate (TMSOTf) were purchased from Ourchem Chemical Co., Ltd (Shanghai, China). Chloroform-d, pyridine-d5 and DMSO-d6 were obtained from Cambridge Isotope Laboratories, Inc. (MA, USA). Acetonitrile and formic acid of LC-MS grade were purchased from Dikma Technologies Inc. (CA, USA). All other analytical chemical reagents of analytical grade were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). Deionized water was purified using a Milli-Q system (MA, USA). Silica gel (FCP, 200–300 Mesh) was used for flash chromatography.Three reference standards timosaponin A-III, timosaponin B-III, TB-II were previously isolated from the Rhizoma Anemarrhenae in our laboratory and their chemical structures were unambiguously identified by comparison of their NMR and MS data with those in previous literature,9–11 the purities were all more than 98% by HPLC determination.
Sample preparation
All animal procedures were conducted in accordance with the guidelines from the Review Committee of Animal Care and Use at the Shanghai Institute of Materia Medica (Shanghai, China). Six male Sprague-Dawley rats (200–220 g; Shanghai SLAC Laboratory Animal Co., Shanghai, China) were kept in an environmentally controlled breeding room for 1 week and fasted 12 h (but with access to water) before starting the experiments.Urine samples were obtained from six rats that have been on treatment with TB-II of 300 mg kg−1 using metabolic cages. A 600 μL aliquot of mixed drug-containing urine samples was loaded onto a Solid Phase Extraction (SPE) cartridge, which was preconditioned with 2 mL methanol and 2 mL water. Then the cartridge was washed with 1 mL of water and the analyte eluted with 1 mL of methanol. The eluted solution was evaporated to dryness in a water bath at 37 °C under a gentle stream of nitrogen and the residue was reconstituted in 200 μL methanol. The resulting solution was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C and 1.0 μL of supernatant was injected into the chromatographic system for LC-MS/MS analysis. Blank samples as controls were prepared with the same method as the drug-containing samples.
000 rpm for 10 min at 4 °C and 1.0 μL of supernatant was injected into the chromatographic system for LC-MS/MS analysis. Blank samples as controls were prepared with the same method as the drug-containing samples.
Instruments and analytical conditions
1H, 13C-NMR spectra and two dimension NMR spectra (HSQC and HMBC) were obtained on Bruker DRX-400 (Bruker, Germany) spectrometer at 26 °C, with DMSO-d6, pyridine-d5 or chloroform-d as the solvents.The chromatography analytical procedures were performed on an Agilent 1260 Series (Agilent, Santa Clara, CA, USA) LC system equipped with a binary pump, an online degasser, an auto plate-sampler, and a thermostatically controlled column compartment. The separation was carried out on an Agilent poroshell 120 EC-C18 column (100 mm × 2.1 mm, 2.7 μm; Agilent, CA, USA). The binary gradient elution system consisted of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B) and separation was achieved using the following gradient: 8–15% B at 0–5 min, 15–24% B at 5–10 min; 24–28% B at 10–15 min; 28–50% B at 15–30 min; 50–90% B at 30–45 min. The composition was then held at 90% B for 5 min and returned to initial conditions and maintained 6 min for equilibration. The flow rate was 0.35 mL min−1 and the injection volume was 1.0 μL.
Mass spectrometry was performed using an Agilent 6530B Q-TOF mass spectrometer (Agilent, Santa Clara, CA, USA) equipped with an electrospray ionization (ESI) interface, and was operated in positive ion mode with parameters set as follows: capillary voltage, 4000 V; fragmentor, 150 V; pressure of nebulizer, 35 psi; drying gas temperature, 300 °C; sheath gas temperature, 350 °C. Nitrogen was used as sheath and drying gas at a flow rate of 8.0 L min−1 and 11.0 L min−1, respectively. Data were collected in centroid mode and the mass range was set at m/z 50–1200 using the extended dynamic range and the collision energy was optimized at 25 V.
Synthesis of some derivates
The details of synthetic procedures for compounds 1–5 and their NMR information are shown in ESI.†Results and discussion
Chemistry
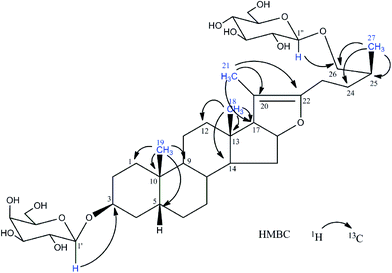
The structures of compounds 1–4 were confirmed by 1H-NMR, 13C-NMR, and HR-MS spectral data. The significant differences between 1 and 2, as well as 3 and 4, are the chemical shifts of H-27 and H-26 because of the configuration of C-25,12,13 that is, as for 25S configuration, the chemical shifts of H-27 were in the lower field than that of 25R (0.98 ppm, J = 6.4 Hz for 25S while 0.77 ppm, J = 6.2 Hz for 25R) and the difference between two protons of H-26 was more distinct (Δ26-H = 0.65 ppm for 25S while Δ26-H = 0.10 ppm for 25R) (Fig. 2).One novel acid hydrolysis product of timosaponin BIII-d was also obtained. Compound 5 was obtained as a white amorphous powder. Its molecular formula, C39H64O13 (m/z 741.4422; calcd for C39H65O13+, 741.4420) was deduced by positive HR-ESI/MS. Furthermore, the ESI-MS/MS (positive-ion mode) of 5 showed the characteristic ion peaks at m/z 741.4 [M + H]+, 579.4 [M + H-162]+, and 417.3 [M + H-162-162]+, suggesting the presence of two hexose units. The 1H and 13C NMR spectral data of 5 (Table 1) were almost identical to those standard compound of timosaponin B-III, possessing the characteristic signals of furostanol saponin such as four methyl signals at δH 0.71 (s, Me-18), 0.85 (s, Me-19), 1.64 (s, Me-21) and 1.04 (d, J = 6.8 Hz, Me-27), two olefinic carbons at δC 152.9 (C-22) and 104.1 (C-20). However, two signals for anomeric protons at δH 4.89 (d, J = 7.6 Hz), 4.84 (d, J = 7.7 Hz) suggested the presence of only two sugar moieties. By analysis of the 1D and 2D NMR spectra, they were identified as β-galactose and β-glucose units, respectively. The HMBC spectrum showed correlations between H-1′ (δH 4.89) of galactose unit and C-3 (δC 75.7), between H-1′′ (δH 4.84) of glucose unit and C-26 (δC 75.8) of the aglycone moiety (Fig. 3). Thus, 2 was determined to be (25S)-26-O-β-D-glucopyranosyl-20(22)-ene-5β-furost-3-O-β-D-galactopyranoside, which was simply named timosaponin BIII-d.
| Position | δC (ppm) | δH (ppm) | Position | δC (ppm) | δH (ppm) |
|---|---|---|---|---|---|
| a Note: (o) overlapped with other signals. | |||||
| 1 | 31.5 | 1.50 (m), 1.47 (o) | 22 | 152.9 | — |
| 2 | 27.3 | 2.02 (m), 1.10 (m) | 23 | 31.1 | 2.07 (m), 1.56 (m) |
| 3 | 75.7 | 4.25 (o) | 24 | 24.1 | 2.26 (m), 1.04 (o) |
| 4 | 34.9 | 2.12 (m), 1.46 (o) | 25 | 34.2 | 1.97 (m) |
| 5 | 37.5 | 2.03 (o) | 26 | 75.8 | 4.05 (o), 3.50 (m) |
| 6 | 27.5 | 1.29 (m), 1.13 (o) | 27 | 17.7 | 1.04 (d, J = 6.8 Hz) |
| 7 | 27.6 | 1.57 (m), 1.09 (o) | Gal | ||
| 8 | 35.7 | 1.46 (o) | 1′ | 104.4 | 4.89 (d, J = 7.6 Hz) |
| 9 | 40.7 | 1.31 (m) | 2′ | 73.2 | 4.49 (m) |
| 10 | 35.7 | — | 3′ | 77.4 | 4.11 (o) |
| 11 | 21.8 | 1.36 (m), 1.21 (o) | 4′ | 70.8 | 4.63 (m) |
| 12 | 40.6 | 1.76 (m), 1.20 (o) | 5′ | 76.0 | 4.20 (o) |
| 13 | 44.4 | — | 6′ | 62.9 | 4.48 (o), 4.11 (o) |
| 14 | 55.2 | 0.88 (o) | Glu | ||
| 15 | 31.9 | 1.87 (m), 1.49 (m) | 1′′ | 105.7 | 4.84 (d, J = 7.7 Hz) |
| 16 | 85.1 | 4.85 (o) | 2′′ | 75.0 | 4.05 (o) |
| 17 | 65.2 | 2.51 (m) | 3′′ | 79.1 | 4.25 (o) |
| 18 | 14.8 | 0.71 (s) | 4′′ | 72.2 | 4.25 (o) |
| 19 | 24.4 | 0.85 (s) | 5′′ | 79.0 | 3.97 (m) |
| 20 | 104.1 | — | 6′′ | 63.3 | 4.58 (m),4.42 (m) |
| 21 | 12.3 | 1.64 (s) | |||
HPLC-MS/MS characterization of the synthetic products and commercial standards
In order to standardize the reference compounds, the retention time and MS spectral data of synthetic products and commercial standards were analyzed. Meanwhile, we have summarized the fragmentation patterns and chromatographic retention behaviors that will be valuable for the subsequent on-line elucidation of structurally related compounds.These data will provide a scientific basis for identification of the metabolites in biological samples. Positive ion mode was selected for HPLC-MS/MS analysis in this study, as it provides extensive structural information via collision-induced dissociation (CID). A typical MS total ion chromatogram (TIC) of eight reference compounds studied in the experiment is shown in ESI, Fig. S13(a).† Positive ESI analysis of S2 gave the [M + H]+ ion at m/z 903.4948 (C45H75O18+, 0 ppm). The MS/MS CID fragmentation of the m/z 903 ion yielded three product ions at m/z 741.4417 (C39H65O13+, −0.4 ppm), 579.3887 (C33H55O8+, −0.7 ppm) and 417.3363 (C27H45O3+, 0 ppm) (Fig. 4a) through consecutive losses of three hexose units (162 Da*3), respectively. The ions at m/z 417 continued to yield the ion at m/z 399.3254 (C27H43O2+, −1.0 ppm) by loss of a H2O (18 Da). In addition, the fragment ion at m/z 273.2208 (C19H29O+, −1.8 ppm) was attributed to a skeleton residue by the cleavage of E-ring due to the presence of a 16,22-epoxy residue and a 22,26-epoxy residue, and then the ion continued to lose a molecule of H2O to yield an ion at m/z 255.2109 (C19H27+, 0.9 ppm). Based on the above data, compound S2 was confirmed as timosaponin B-III. The proposed fragmentation pathway for compound S2 is shown in Fig. 4b.
 | ||
| Fig. 4 Representative MS/MS spectrum of timosaponin BIII (a) and timosaponin A-I (c); the proposed fragmentation pathway of timosaponin BIII (b). | ||
Compound S5 yielded the [M + H]+ ion at m/z 579.3892 (C33H55O8+, 0.2 ppm). And as the S2 did, its MS/MS spectra produced four prominent fragment ions at m/z 417.3362, 399.3253 (low), 273.2215 and 255.2107 (Fig. 4c), originating from losses of hexose unit (162 Da), H2O (18 Da) and E-ring cleavage, respectively. However, there were two compounds (timosaponin A-I and its isomer) with the elementary composition of C33H54O8 among the eight reference compounds, compound S5 was ultimately identified as isotimosaponin A-I by a single injection analysis (ESI, Fig. S13(c)†), and the compound S6 was confirmed as timosaponin A-I. Similarly, the isomers (S3 & S4 and S7 & S8) were successively differentiated as shown in Fig. S13(b) and (d).†
Taking the same identification methods described above, the eight reference compounds were unambiguously identified as TB-II, timosaponin B-III, timosaponin BIII-d, timosaponin A-III, isotimosaponin A-I, timosaponin A-I, isosarsasapogenin, and sarsasapogenin respectively. The retention time, ESI-MS/MS data and fragmentations of eight standard compounds are summarized in Table 2.
| Peak no. | tR (min) | Formula | Experimental massa (m/z) | Calculated Mass (m/z) | Errorb (ppm) | Errorc (mDa) | MS/MS fragments (m/z) | Proposed compound |
|---|---|---|---|---|---|---|---|---|
| a Gave [M + H]+ as quasi-molecular ions.b Differences between the measured and calculated values.c Milli-dalton, differences between the measured and calculated values.d Identified with the reference compounds. | ||||||||
| 1 | 14.285 | C33H52O9 | 593.3688 | 593.3684 | 0.7 | 0.4 | 417.3366, 273.2211, 255.2108 | Sarsasagenin/isosarsasapogenin glucuronide |
| 2 | 16.165 | C45H76O19 | 921.5055 | 921.5054 | 0.1 | 0.1 | 903.4952, 741.4415, 579.3883, 417.3363, 399.3262, 273.2205, 255.2113 | TB-IId |
| 3 | 22.009 | C45H74O18 | 903.4948 | 903.4948 | 0 | 0 | 741.4417, 579.3887, 417.3363, 399.3261, 273.2208, 255.2109 | Timosaponin BIIId |
| 4 | 23.280 | C39H66O14 | 759.4521 | 759.4525 | −0.5 | −0.4 | 741.4420, 579.3889, 417.3362, 399.3253, 273.2211, 255.2115 | Deglycosylated TB-II |
| 5 | 25.068 | C39H64O13 | 741.4422 | 741.4420 | 0.3 | 0.2 | 579.3890, 417.3373, 273.2207, 255.2103 | Timosaponin BIII-dd |
| 6 | 27.142 | C33H56O9 | 597.4001 | 597.3997 | 0.7 | 0.4 | 579.3899, 417.3371, 273.2214, 255.2101 | Di-deglycosylated TB-II |
| 7 | 27.955 | C33H54O8 | 579.3899 | 579.3891 | 1.4 | 0.8 | 417.3370, 399.3266, 273.2216, 255.2112 | Deglycosylated timosaponin BIII-d |
| 8 | 30.902 | C39H64O13 | 741.4422 | 741.4420 | 0.3 | 0.2 | 579.3894, 417.3373, 399.3269, 273.2218, 255.2110 | Isotimosaponin AIII |
| 9 | 32.935 | C39H64O13 | 741.4411 | 741.4420 | −1.2 | −0.9 | 579.3884, 417.3352, 399.3254, 273.2209, 255.2101 | Timosaponin AIIId |
| 10 | 38.068 | C33H54O8 | 579.3892 | 579.3891 | 0.2 | 0.1 | 417.3362, 273.2215, 255.2107 | Isotimosaponin AId |
| 11 | 38.423 | C33H54O8 | 579.3895 | 579.3891 | 0.7 | 0.4 | 417.3360, 273.2211, 255.2109 | Timosaponin AId |
| 12 | 44.979 | C27H44O3 | 417.3367 | 417.3363 | 1.0 | 0.4 | 273.2213, 255.2103 | Isosarsasapogenind |
| 13 | 47.011 | C27H44O3 | 417.3372 | 417.3363 | 2.2 | 0.9 | 273.2217, 255.2112 | Sarsasapogenind |
The ESI-MS/MS data of these compounds shared some common features, such as the neutral losses of terminal hexose units (162 Da), H2O (18 Da), and the partial cleavage of E-ring. It could be inferred that the ions at m/z 273 (C19H29O+) and 255 (C19H27+) were a pair of characteristic product ions of timosaponin and they could be used for structural elucidation of these derivatives with similar skeletons. Furthermore, we could tentatively conclude that the retention time of spirostanols were longer than furostanols on the reversed phase liquid chromatography (RPLC) column, and 25S was longer than that of 25R.
Identification of the metabolites in rat urine by HPLC-MS/MS
Excluding the influence of endogenous matrix in the biological sample, 13 peaks were tentatively predicted to be parent compound or metabolites of TB-II by comparing drug-containing samples with the corresponding control samples directly (Fig. 5). By comparing the accurate masses of peaks appearing in the chromatograms of drug-containing urine with those previously identified reference compounds, all the eight reference compounds (peaks 2, 3, 5, 9, 10, 11, 12 and 13) in urine were found, namely one parent compound and seven metabolites could be identified unambiguously. Besides, another five possible metabolites were also inferred according to the obtained exact molecular weight and its MS/MS information. | ||
| Fig. 5 Total ion chromatograms (TICs) for rat blank urine (a) and urine after oral administration of TB-II (b) in positive mode. | ||
Peak 1 eluted at 14.285 min, giving the [M + H]+ ion at m/z 593.3688 (C33H53O9+, 0.7 ppm), which was 176 Da higher than that of sarsasapogenin/isosarsasapogenin. After losing a neutral fragment 176 Da (a glucuronide unit), the fragment ions at m/z 417.3366, 273.2211 and 255.2108 were the same as those of sarsasapogenin/isosarsasapogenin, suggesting that peak 1 may be the glucuronide conjugate of sarsasapogenin/isosarsasapogenin. As for peaks 4 and 6, the molecular ions of peak 4 (m/z 759.4521) and peak 6 (m/z 597.4001) were 162 and 324 Da less than that of TB-II, respectively. Furthermore, the representative fragment ions also showed no difference with TB-II. Therefore, they were tentatively identified as deglycosylated TB-II and di-deglycosylated TB-II, respectively. Similarly, peak 7 could be tentatively identified as deglycosylated timosaponin BIII-d.
Peak 8 exhibited the same [M + H]+ ion with peaks 5 and 9 at m/z 741.4422 (C39H65O13+, 0.3 ppm), revealing that these three compounds were isomers. Moreover, they all showed the same fragments ions in the MS/MS spectra. On the basis of analysis of peaks 10 and 11, as well as peaks 12 and 13, we could infer that there may be an isomer of timosaponin A-III, which were isomerized only at C-25 in the process of metabolism in vivo. Therefore, peak 8 was tentatively identified as isotimosaponin A-III.
Conclusions
In this study, 13 metabolites in urine were detected by HPLC-Q-TOF-MS after an oral administration of TB-II to rats, and eight of them were identified unambiguously by the reference compounds, the proposed metabolic pathway of TB-II in rats is presented in Fig. 6. The metabolic pathways of TB-II were mainly deglycosylation and F-ring isomerization. Meanwhile, it is so interesting that the furostanol timosaponin mainly transform to spirostanol and even the isospirostanol timosaponin (configuration of C-25 will convert to R) in vivo. To the best of our knowledge, it is the first report of the isomerization behaviors. As described in the previous literature,14–16 the compounds of smilagenin (isosarsasapogenin) and 25R spirostanol timosaponin have wide range of pharmacological activities, such as stimulating the gene expression of BDNF, improving memory, and antitumor activities. This means that the biological activity of TB-II could be attributed to the presence of the metabolites with stereoisomerism at C25. The results in this work are important for the understanding of TB-II metabolism in rats and provide useful information and reference for similar study in humans.Acknowledgements
Financial support for this research was provided by grant from the Key Program and General Program of National Natural Science Foundation of China (no. 81030065 and 81274055).References
- Y. Hu, Z. Q. Xia, Q. X. Sun, A. Orsi and D. Rees, Brain Res., 2005, 1060, 26–39 CrossRef CAS PubMed.
- T. J. Li, Y. Qiu, P. Y. Yang, Y. C. Rui and W. S. Chen, Neurosci. Lett., 2007, 421, 147–151 CrossRef CAS PubMed.
- W. Q. Lu, Y. Qiu, T. J. Li, X. Tao, L. N. Sun and W. S. Chen, Arch. Pharmacal Res., 2009, 32, 1301–1308 CrossRef CAS PubMed.
- S. Ouyang, L. S. Sun, S. L. Guo, X. Liu and J. P. Xu, Acad. J. First Med. Coll. PLA, 2005, 25, 121–126 CAS.
- P. Williams, A. Sorribas and M.-J. R. Howes, Nat. Prod. Rep., 2011, 28, 48–77 RSC.
- F. Cai, L. N. Sun, S. H. Gao, Y. Yang, Q. Yang and W. S. Chen, J. Pharm. Biomed. Anal., 2008, 48, 1411–1416 CrossRef CAS PubMed.
- F. Cai, W. Xu, H. Wei, L. N. Sun, S. H. Gao, Q. Yang, J. Feng, F. Zhang and W. S. Chen, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2010, 878, 1845–1854 CrossRef CAS PubMed.
- Z. R. Liu, D. L. Zhu, L. Lv, Y. Y. Li, X. Dong, Z. Y. Zhu and Y. F. Chai, Rapid Commun. Mass Spectrom., 2012, 26, 1955–1964 CrossRef CAS PubMed.
- S. H. Cheng, Y. G. Du, B. P. Ma and D. W. Tan, Org. Biomol. Chem., 2009, 7, 3112–3118 CAS.
- Y. Peng, Y. J. Zhang, Z. Q. Ma, W. San Pan, Y. Q. Sun and S. J. Song, Chin. Chem. Lett., 2007, 18, 171–174 CrossRef CAS PubMed.
- T. Kawasaki and T. Yamauchi, Chem. Pharm. Bull., 1963, 11, 1221 CrossRef CAS.
- A. Tobari, M. Teshima, J. Koyanagi, M. Kawase, H. Miyamae, K. Yoza, A. Takasaki, Y. Nagamura and S. Saito, Eur. J. Med. Chem., 2000, 35, 511–527 CrossRef CAS.
- P. K. Agrawal, Steroids, 2005, 70, 715–724 CrossRef CAS PubMed.
- Y. Hu, Z. M. Wang, R. Zhang, P. P. Wu, Z. Q. Xia, A. Orsi and D. Rees, Neurobiol. Aging, 2010, 31, 1010–1019 CrossRef CAS PubMed.
- R. Zhang, Z. Wang, P. A. Howson, Z. Xia, S. Zhou, E. Wu, Z. Xia and Y. Hu, Neuroscience, 2012, 210, 275–285 CrossRef CAS PubMed.
- J. Eskander, O. K. Sakka, D. Harakat and C. Lavaud, Med. Chem. Res., 2013, 22, 4877–4885 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09133k |
| ‡ These two authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2015 |