An electrochemical chiral sensor for tryptophan enantiomers based on reduced graphene oxide/1,10-phenanthroline copper(II) functional composites
Hao Goua,
Jingxian Heb,
Zunli Mo*a,
Xiaojiao Weia,
Rere Hua and
Yawei Wanga
aKey Laboratory of Eco-Environment-Related Polymer Materials, Ministry of Education of China, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou 730070, PR China. E-mail: mozlnwnu2010@163.com
bSchool of Chemistry and Environmental Science, Lanzhou City University, Lanzhou 730070, PR China
First published on 2nd July 2015
Abstract
An electrochemical chiral sensor based on reduced graphene oxide (RGO) non-covalently functionalized with 1,10-phenanthroline copper(II) (PhenCu) complex has been developed for electrochemical discrimination of tryptophan (Trp) enantiomers. The formation and morphology of reduced graphene oxide/1,10-phenanthroline copper(II) (RGO/PhenCu) composites were confirmed by Fourier-transform infrared spectroscopy (FT-IR) and scanning electron microscopy (SEM). Cyclic voltammetry (CV) was employed to monitor the electrochemical behavior of RGO/PhenCu immobilized on glassy carbon electrode (RGO/PhenCu/GCE). The reduction in peak current was significantly different when the chiral sensor interacted with L-Trp and D-Trp. This suggested that RGO/PhenCu/GCE could be used as an electrochemical chiral sensor for the discrimination of Trp enantiomers. Further studies showed that the peak current decreased linearly along with an increasing percentage of L-Trp in the Trp mixture. The RGO/PhenCu/GCE electrochemical chiral sensor, with rapid recognition, good sensitivity and high stability, provided an efficient method to recognize and discriminate Trp enantiomers.
1. Introduction
Chiral sensors are important from the viewpoint of medical care and pharmaceutical technologies because most biologically relevant molecules possess chirality.1,2 However, the enantiomers of these chiral molecules often exhibit remarkable discrepancies in terms of biochemical activity, toxicity, potency, metabolic pathway and so on.3 Recently, chiral discrimination of biological molecules, such as carbohydrates, peptides and amino acids, has attracted increasing interest in the biochemical and pharmaceutical fields.4–6 Traditional techniques for discriminating enantiomers are usually based on high performance liquid chromatography, chemiluminescence and capillary electrochromatography.7 Although these techniques are useful for the determination of enantiomeric purity, they not only require expensive chiral columns and complex sample pretreatment, but also many of them indicate no difference between the two enantiomeric forms of some amino acids.8 In comparison, electrochemical methods can be evaluated as potential enantiomeric analysis techniques due to their advantages of high stability and sensitivity, low cost, and rapid detection.9Tryptophan enantiomers, which have an asymmetric carbon in their structure, play a very important role in biological systems. As an essential amino acid, Trp has been determined to be a precursor of the neurotransmitter serotonin, and the level of Trp in plasma is closely related to the extent of hepatic disease.10 Therefore, it is very important to develop an alternative method for chiral discrimination of Trp enantiomers to meet both general and practical challenges. Several strategies have been reported for the discrimination of Trp enantiomers, including a time-resolved fluorescence technique,11 a potential-induced technique12 and electrode column technology.13 Despite these tremendous achievements, the realization of low-cost, facile and sensitive recognition of Trp enantiomers is still challenging.
Graphene, a single layer of sp2 bonded carbon atoms in a honeycomb two-dimensional lattice, has attracted intense interest as an electrochemical sensing material owing to its extraordinary physical and chemical properties.14 Meanwhile, noticeable progress has also been made in the utilization of graphene-based composites for applications in electrochemical analysis. For example, a graphene/Pt-modified glassy carbon electrode was created to simultaneously characterize ascorbic acid, dopamine, and uric acid levels.15 Deng et al. developed a novel 1,4-bis-(4-aminophenylethynyl)benzene/graphene nanocomposite-modified electrode and successfully demonstrated its use for dopamine determination in human serum samples.16 Wu et al. synthesized a new type of porphyrin-functionalized graphene and used it for highly selective and sensitive detection of dopamine.17 Recently, Asadian et al. produced a graphene nanoribbon/polyaniline composite film for application in the electrochemical determination of dobutamine, and it showed impressive performance.18 Thus it can be seen that graphene-based materials have been used in electrochemical sensors and biosensors. A graphene layer immobilized on an electrode surface not only provides an effective sensing platform but also separates the analytical signal.8 To the best of our knowledge, however, few studies have been done on the application of graphene, and especially graphene modified with selective materials to form a functional composite, in electrochemical detection of chiral molecules.
Herein, novel and stable reduced graphene oxide/1,10-phenanthroline copper(II) functional composites were synthesized based on self-assembly through non-covalent interactions. Non-covalent modifications of graphene will not destroy its intrinsic structure, thus its excellent properties can be preserved. Modification via non-covalent interactions affords a variety of functional composite materials with enhanced mechanical properties, tunable electrical conductivity and potential applications in solar cells, electronic devices and sensing.19 Under the non-covalent interaction model, the aromatic ring of reactants is inclined to be parallel and close to the sp2 network of graphene, and hence the reaction is easy to achieve.20 We found that the PhenCu needle-like structures are uniformly distributed on the surface of reduced graphene oxide to form graphene-based composites, which have potential applications in various fields. The composites showed good capacitive performance and excellent chiral recognition performance when immobilized on GCE. The stepwise fabrication process is illustrated schematically in Scheme 1. In addition, the RGO/PhenCu composites adhere well to the glassy carbon electrode surface by dispersion of the powder in absolute ethanol. This process does not require any adhesive such as nafion, and this makes the electrochemical chiral sensor more economic and environmentally friendly. Since the coordination capacity of Trp enantiomers and 1,10-phenanthroline is different, highly sensitive and selective electrochemical discrimination of Trp enantiomers was realized via ligand exchange. Moreover, the enantioselectivity of RGO/PhenCu/GCE was systematically studied with the variation of incubation time and pH. The reduced graphene oxide/1,10-phenanthroline copper(II) functional composites may provide an alternative approach for the design of novel electrochemical chiral sensors with superior performance.
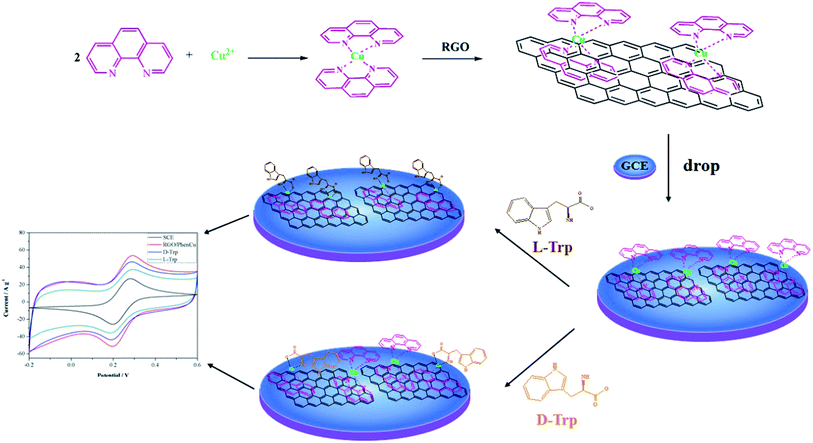 | ||
| Scheme 1 Schematic diagram of RGO/PhenCu/GCE synthesis and the electrochemical response of the developed electrode interacting with Trp enantiomers. | ||
2. Experimental
2.1 Preparation of 1,10-phenanthroline copper(II) complex
Methyl alcohol was added to distilled water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) to form a mixed solution. 1,10-Phenanthroline (50 mM) and CuCl2·2H2O (50 mM) were added to the above solution, followed by stirring at room temperature for 30 min. The blue solution gradually turned blue-green and became cloudy. The solution was filtered and washed 3 to 4 times with distilled water. The resulting blue-green solid was dried at 60 °C for 12 h to give PhenCu.
1 v/v) to form a mixed solution. 1,10-Phenanthroline (50 mM) and CuCl2·2H2O (50 mM) were added to the above solution, followed by stirring at room temperature for 30 min. The blue solution gradually turned blue-green and became cloudy. The solution was filtered and washed 3 to 4 times with distilled water. The resulting blue-green solid was dried at 60 °C for 12 h to give PhenCu.
2.2 Synthesis of RGO/PhenCu
Graphite oxide (GO) was synthesized from graphite powder by an improved method,21 and reduced graphene oxide was obtained by reduction of GO. RGO/PhenCu was prepared by the following method: 0.02 g of RGO was exfoliated in 100 mL of absolute alcohol with ultrasonic treatment for 1 h to form a black solution. PhenCu (0.06 g) was added to the RGO solution and the mixture was stirred at 30 °C for 24 h. The obtained composites were filtered and washed with water and absolute alcohol several times, and dried at 50 °C for 24 h.2.3 Preparation of the RGO/PhenCu/GCE chiral sensor as a working electrode
The surface of GCE was polished carefully with 1.0, 0.3 and 0.05 μm alumina slurry in turn, and rinsed with doubly distilled water, followed by cleaning by sonication in ethanol solution and doubly distilled water consecutively, and then dried in air.The RGO/PhenCu composites (1 mg mL−1) were dispersed in absolute alcohol and sonicated for 30 min. Then, a drop of the suspension (5 μL) was cast onto the freshly polished GCE and dried in air at room temperature.
2.4 Reagents and apparatus
All chemicals were of analytical grade and used without further purification. Graphite powder (99.99%), L-Trp, D-Trp and 1,10-phenanthroline were obtained from Aladdin Industrial Corporation. Concentrated H2SO4, H2O2, KMnO4, K3[Fe(CN)6] and K4[Fe(CN)6]·3H2O were supplied by Tianjin Kaixin Chemical reagents Co., Ltd. NaH2PO4·2H2O, Na2HPO4·12H2O, KNO3 and CuCl2·2H2O were purchased from Tianjin Kaitong Chemical Plant reagents Co., Ltd.Scanning electron microscopy images were obtained using a JSM-6701F cold field emission scanning electron microscope (Japan). Fourier-transform infrared spectra were recorded on an EQUINOX55 FT-IR spectrometer with KBr pellets. Cyclic voltammetry (CV) measurements were performed with a CHI 660E electrochemistry workstation (Shanghai Chenhua Instruments Co., China).
2.5 Experimental measurements
All electrochemical experiments were carried out with a three-electrode system, in which either the bare or modified glassy carbon electrode was used as the working electrode, a saturated calomel electrode was used as the reference electrode and a platinum wire was used as the auxiliary electrode. The CV scan was carried out from 0.2 to 0.6 V at a rate of 50 mV s−1 in 5 mM [Fe(CN)6]4−/3− solutions of varying pH, which were prepared with phosphate buffer solution (PBS), and using 0.1 M KNO3 as the supporting electrolyte.The detection was based on the difference in oxidation peak current (ΔI) before and after the RGO/PhenCu/GCE chiral sensor was immersed into a 5 mM L-Trp or D-Trp solution. ΔI was calculated using the equation ΔI = I0 − I1, where I0 is the oxidation peak current of the RGO/PhenCu/GCE chiral sensor, and I1 is the oxidation peak current after the chiral sensor has interacted with the Trp enantiomer solution. ΔI′ = I′0 −I′1, where I′0 is the oxidation peak current after the chiral sensor has interacted with D-Trp, and I′1 is the oxidation peak current after the chiral sensor has interacted with L-Trp.
3. Results and discussion
3.1 Characterization of the composites
Fig. 1 shows the Fourier-transform infrared spectra of Phen, PhenCu, reduced graphene oxide, and the RGO/PhenCu composites. Compared with the FT-IR spectrum of Phen (Fig. 1a), in the spectrum of PhenCu (Fig. 1b), the characteristic peaks of 1,10-phenanthroline appear, and especially the peaks at 1523 cm−1, 867 cm−1 and 717 cm−1 are red-shifted after the formation of PhenCu. In addition, all the characteristic peaks become weaker. The above analysis indicates coordination of Cu2+ and the 1,10-phenanthroline. As shown in Fig. 1b–d, the FT-IR spectrum of the RGO/PhenCu composites exhibits the respective absorption features of RGO and PhenCu, and the peaks become weak and shifted. This result confirms that the PhenCu was non-covalently grafted onto the surface of the reduced graphene oxide sheet successfully. | ||
| Fig. 1 FT-IR spectra of (a) 1,10-phenanthroline, (b) 1,10-phenanthroline copper(II), (c) reduced graphene oxide, and (d) the RGO/PhenCu composites. | ||
The non-covalent modification of reduced graphene oxide by PhenCu was further confirmed by scanning electron microscopy. As can be seen in Fig. 2a, the reduced graphene oxide appeared to be of good quality with large size and thickness, and the surface is glazed and wrinkled, resembling silk or satin. From Fig. 2b, the morphology of the RGO/PhenCu composites is substantially different from that of reduced graphene oxide. It can be clearly seen that the PhenCu needle-like structures are uniformly distributed on the surface of reduced graphene oxide to form reduced graphene oxide-based composites. Large-scale SEM images showed that the sizes of the PhenCu needle-like structures are about 1–2 μm (Fig. 2c). Nevertheless, the PhenCu complexes eventually become amorphous structures after growing naturally for 3 months without any processing, as shown in Fig. 2d. In contrast, the RGO/PhenCu composites become more orderly; this may be because reduced graphene oxide provides a strong basis for growth of the PhenCu complex. On the other hand, the PhenCu complex can also grow by itself due to intermolecular forces between 1,10-phenanthroline ligands. The complex grows along the peaks and troughs of reduced graphene oxide into needle-like structures, and the erect part looks like a peeled pomelo. This structure gives two advantages: one is increasing the contact area between the composites and enantiomers, which could promote the coordination reaction, and the other is that reduced graphene oxide provides an effective sensing platform and greatly improves the detection sensitivity for chiral tryptophan.
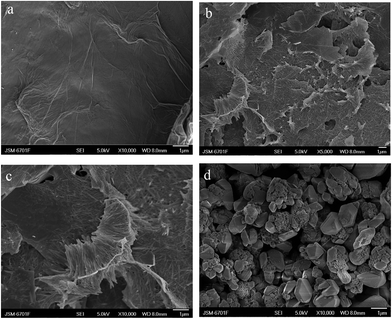 | ||
| Fig. 2 The composition and morphology of (a) reduced graphene oxide, (b) and (c) RGO/PhenCu, and (d) PhenCu. | ||
3.2 Electrochemical characterization of the composites
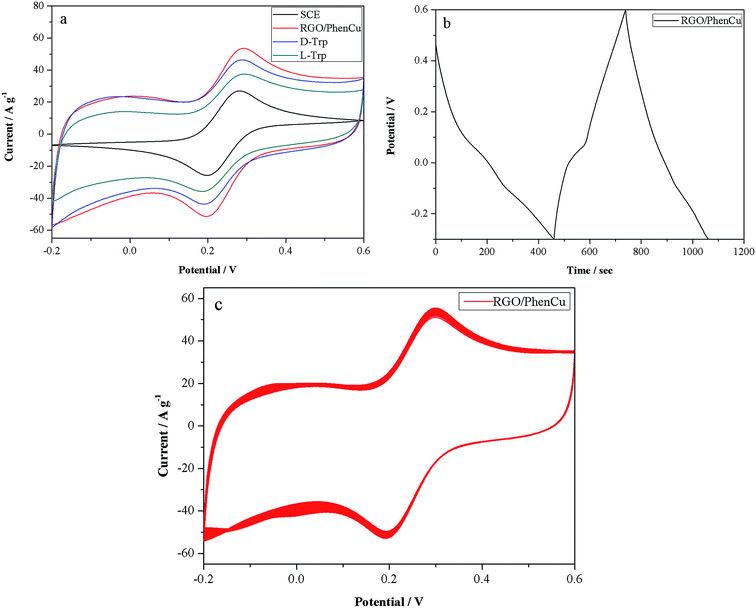
We studied the electrochemical and sensing performance of the RGO/PhenCu composites by cyclic voltammetry and galvanostatic charge–discharge tests. Fig. 3a shows the CV curves of SCE and RGO/PhenCu composites at a scan rate of 50 mV s−1. The integral area of the RGO/PhenCu composites is much larger than that of SCE, and the CV curve of the composites shows a well-defined and reversible redox peak and reduction peak, which indicates that the redox reaction has good reversibility and a fast charge transfer process. Fig. 3b shows galvanostatic discharge curves of RGO/PhenCu composites at 1 A g−1. The specific capacitance is calculated to be 362 F g−1 at a current density of 1 A g−1, which is higher than pure reduced graphene oxide. It is worth mentioning that the charge/discharge curves are very symmetrical and an obvious plateau at low potentials can be observed. The plateau is related to the quick Faradaic reactions of the RGO/PhenCu composites, which is in agreement with the CV curves. The cycle durability is an important concern for capacitors. As seen in Fig. 3c, after 200 cycles at 50 mV s−1, the curve remained nearly unchanged, indicating excellent electrochemical stability of the RGO/PhenCu composites. This study showed that RGO/PhenCu composites could be expected to be supercapacitors.3.3 Enantioselective discrimination of Trp enantiomers
More importantly, the electrochemical response before and after the interaction of RGO/PhenCu/GCE with L-Trp and D-Trp has been investigated by CV. As can be clearly seen in Fig. 3a, the peak current decreased after the chiral sensor was dipped in 5 mM Trp enantiomer solution for 6 min. However, an obvious chiral discrepancy was noticed, where a larger decrease was obtained from the interaction of RGO/PhenCu/GCE and L-Trp (the current declined by 16.06 A g−1) than that of RGO/PhenCu/GCE and D-Trp (the current declined by 7.16 A g−1). That is, the total amount of chiral ligand exchange existing between RGO/PhenCu and L-Trp was larger than for D-Trp. The main reaction is described as follows:| L-Trp + [Cu(II)(Phen)2] = [(L-Trp)Cu(II)(Phen)] + Phen |
| D-Trp + [Cu(II)(Phen)2] = [(D-Trp)Cu(II)(Phen)] + Phen |
That is to say, the coordination capacity of Trp enantiomers and Cu(II) is stronger than that of Phen, and L-Trp can exchange with one Phen to form [(L-Trp)Cu(II)(Phen)] more easily. In this reaction process, the perfectly ordered needle-like structure of RGO/PhenCu composites can increase the contact area between PhenCu and the Trp enantiomers, which accelerates the rate of chiral ligand exchange, thus shortening the measurement time and improving sensing test efficiency. Therefore, RGO/PhenCu/GCE could discriminate between the Trp enantiomers.
3.4 The influence of the amounts of Trp enantiomers
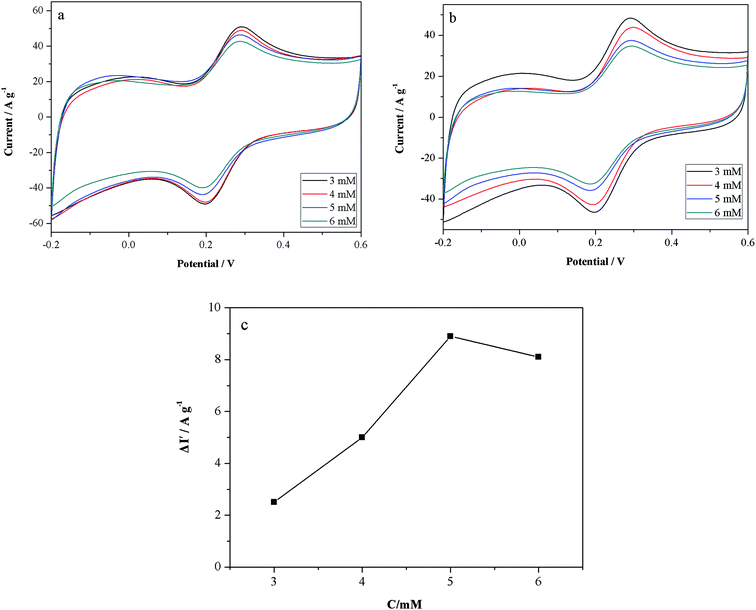
In order to gain an insight into the relationship between the peak current and the concentration of Trp, and to find the best testing concentration of Trp enantiomers, additional CV measurements were performed in the presence of different concentrations of D-Trp and L-Trp. We found that the peak current of D-Trp decreased with an increasing amount of D-Trp in the range 3–6 mM, as shown in Fig. 4a. The reduction of peak current from 3 mM to 5 mM is slow and uniform. This change becomes greater when the D-Trp concentration increases to 6 mM. This is mainly because only small amounts of D-Trp can react with PhenCu, and the effective concentration of [Fe(CN)6]4−/3− falls after adding the D-Trp. The peak current of L-Trp also decreased with an increasing concentration of L-Trp in the range 3–6 mM, as shown in Fig. 4b. It is important to note that although D-Trp and L-Trp both show a decrease in peak current with increasing Trp enantiomer concentration, L-Trp shows a more obvious change than D-Trp. This means that a larger amount of L-Trp undergoes the ligand exchange reaction with PhenCu. It also means that L-Trp has a stronger affinity to PhenCu than D-Trp. As shown in Fig. 4c, ΔI′ had the highest value when the concentration of Trp enantiomers was 5 mM. This shows that the reaction with L-Trp is maximized when the amount of RGO/PhenCu on the GCE is kept constant. Therefore, 5 mM was chosen as the concentration for further tests.3.5 The influence of interaction time
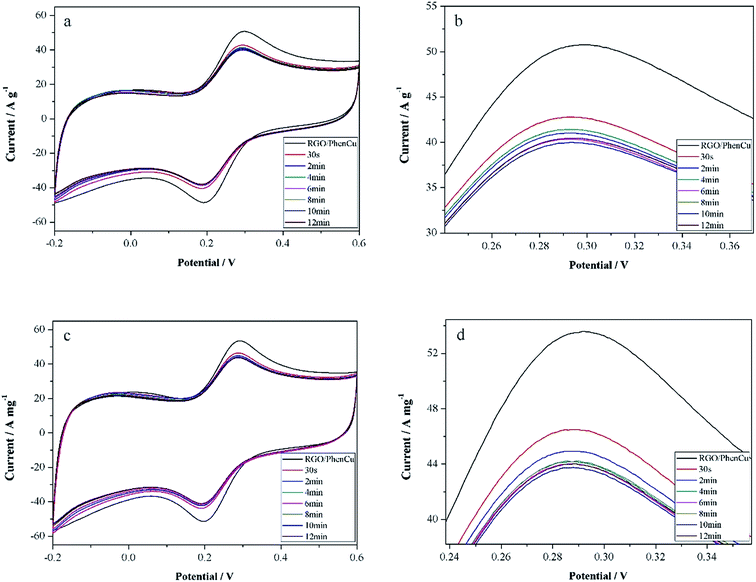
The influence of reaction time on the enantioselective interaction was studied between 30 s and 12 min. As shown in Fig. 5, the peak current decreased with prolongation of the reaction time. No further decrease is evident after 6 min for either L-Trp or D-Trp, and ΔI remained at maximum after the interaction time was increased to 6 min. Therefore, 6 min was adopted as the best recognition time in this work. This value is smaller than previously reported,22–24 which raises the detection efficiency significantly.3.6 The influence of pH
The influence of pH on the enantioselective discrimination was investigated in the pH range from 5.3 to 7.0 (Fig. 6). However, we found that ΔI was smaller after PBS addition. It can be seen that the maximum difference in peak current density appeared without PBS. The reason may be that the addition of PBS slows the ligand exchange reaction between RGO/PhenCu/GCE and the Trp enantiomers. Therefore, all subsequent experiments were performed without PBS.3.7 Application of the chiral sensor
The RGO/PhenCu/GCE was also used to detect the current responses of Trp enantiomeric mixtures at different fixed ratios. The standard calibration curves and cyclic voltammograms of Trp enantiomeric mixtures are presented in Fig. 7. It is clear that the peak current decreased uniformly as the amount of L-Trp increased. Moreover, the correlation between ΔI and L-Trp% exhibited a linear relationship. The linear regression can be expressed as ΔI = 0.09171 × L-Trp% + 7.04443 (R2 = 0.99268). To the best of our knowledge, such good chiral recognition ability is comparable or superior to the best results reported in the literature, as shown in Table 1. By comparison, this electrochemical chiral sensor is more convenient, highly efficient and sensitive. These results demonstrate that the RGO/PhenCu/GCE electrode provides an opportunity for not only higher chiral recognition ability for Trp enantiomeric mixtures, but also for the determination of one enantiomer in the presence of the other. | ||
| Fig. 7 The linear calibration curve for the percentage of L-Trp. Inset: enlarged image of the cyclic voltammograms of Trp enantiomeric mixtures. | ||
4. Conclusion
In summary, novel PhenCu-functionalized reduced graphene oxide composites were successfully synthesized. The composites possess distinctive needle-like structures, which provide potential applications in various fields. For example, they have good capacitive performance. More importantly, the RGO/PhenCu composites have been fabricated for discrimination of Trp enantiomers when immobilized on GCE. Reduced graphene oxide with high electron mobility and a large surface area immobilized on GCE is expected not only to provide an effective sensing platform but also to improve the detection sensitivity. Its special structure is helpful for increasing the contact area between the composites and the enantiomers. Therefore, a RGO/PhenCu/GCE chiral sensor with high sensitivity and rapid detection has been developed to recognize Trp enantiomers by ligand exchange. Moreover, the chiral sensor is very simple, convenient, economical and highly efficient. This study could promote an understanding of competitive reactions between coordination compounds. Besides, it also provides a simple and efficient method to discriminate various chiral enantiomers via electrochemical reactions.Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (51262027), the Natural Science Foundation of Gansu Province (0803RJZA009), Science and Technology Tackle Key Problem Item of Gansu Province (2GS064-A52-036-08) and Gansu Key Laboratory of Polymer Materials (ZD-04-14), and the support from the fund of the State Key Laboratory of Solidification Processing in NWPU (SKLSP201011).References
- Y. F. Bu, S. Wang, Q. Chen, H. L. Jin, J. J. Lin and J. C. Wang, Electrochem. Commun., 2012, 16, 80–83 CrossRef CAS PubMed.
- M. Trojanowicz, Electrochem. Commun., 2014, 38, 47–52 CrossRef CAS PubMed.
- L. Zhang, C. L. Xu, C. W. Liu and B. X. Li, Anal. Chim. Acta, 2014, 809, 123–127 CrossRef CAS PubMed.
- B. M. Wei, N. N. Liu, J. T. Zhang, X. W. Ou, R. X. Duan, Z. K. Yang, X. D. Lou and F. Xia, Anal. Chem., 2015, 87, 2058–2062 CrossRef CAS PubMed.
- L. Taujenis, V. Olšauskaitě and A. Padarauskas, J. Agric. Food Chem., 2014, 62, 11099–11108 CrossRef CAS PubMed.
- R. F. Tiphaine, A. Nathalie, G. Laurence, J. F. Goossens and C. Danel, Carbohydr. Polym., 2015, 115, 598–604 CrossRef PubMed.
- M. Li, X. Liu, F. Y. Jiang, L. P. Guo and L. Yang, J. Chromatogr. A, 2011, 1218, 3725–3729 CrossRef CAS PubMed.
- E. Zor, I. H. Patir, H. Bingol and M. Ersoz, Biosens. Bioelectron., 2013, 42, 321–325 CrossRef CAS PubMed.
- R. Q. Nie, X. J. Bo, H. Wang, L. J. Zeng and L. P. Guo, Electrochem. Commun., 2013, 27, 112–115 CrossRef CAS PubMed.
- Y. X. Tao, J. Y. Dai, Y. Kong and Y. Sha, Anal. Chem., 2014, 86, 2633–2639 CrossRef CAS PubMed.
- Y. L. Wei, S. F. Wang, S. M. Shuang and C. Dong, Talanta, 2010, 81, 1800–1805 CrossRef CAS PubMed.
- Y. Kong, J. X. Wei, W. C. Wang and Z. D. Chen, Electrochim. Acta, 2011, 56, 4770–4774 CrossRef CAS PubMed.
- W. D. Laurentis, L. Khim, J. L. R. Anderson, A. Adam, R. S. Phillips, S. K. Chapman, K. H. van Pee and J. H. Naismith, Biochemistry, 2007, 46, 12393–12404 CrossRef PubMed.
- H. F. Javier, P. Laporta, F. Gutiérrez, M. D. Rubianes, G. Rivas and M. Martínez, Electrochem. Commun., 2014, 39, 26–29 CrossRef PubMed.
- C. L. Sun, H. H. Lee, J. M. Yang and C. C. Wu, Biosens. Bioelectron., 2011, 26, 3450–3455 CrossRef CAS PubMed.
- J. H. Deng, M. L. Liu, F. B. Lin, Y. Y. Zhang, Y. Liu and S. Z. Yao, Anal. Chim. Acta, 2013, 767, 59–65 CrossRef CAS PubMed.
- L. Wu, L. Y. Feng, J. S. Ren and X. G. Qu, Biosens. Bioelectron., 2012, 34, 57–62 CrossRef CAS PubMed.
- E. Asadian, S. Shahrokhian, A. I. Zad and E. Jokar, Sens. Actuators, B, 2014, 196, 582–588 CrossRef CAS PubMed.
- X. Q. Ji, L. Cui, Y. H. Xu and J. Q. Liu, Compos. Sci. Technol., 2015, 106, 25–31 CrossRef CAS PubMed.
- N. An, F. H. Zhang, Z. A. Hu, Z. M. Li, L. Li, Y. Y. Yang, B. S. Guo and Z. Q. Lei, RSC Adv., 2015, 5, 23942–23951 RSC.
- Z. L. Mo, H. Gou, J. X. He, P. P. Yang, C. Feng and R. B. Guo, Appl. Surf. Sci., 2012, 258, 8623–8628 CrossRef CAS PubMed.
- Q. Chen, J. Zhou, Q. Han, Y. H. Wang and Y. Z. Fu, Colloids Surf., B, 2012, 92, 130–135 CrossRef CAS PubMed.
- Q. Zhang, L. J. Guo, Y. H. Huang, Y. H. Wang, Q. Han and Y. Z. Fu, Anal. Methods, 2013, 5, 4397–4401 RSC.
- L. J. Guo, Q. Zhang, Y. H. Huang, Q. Han, Y. H. Wang and Y. Z. Fu, Bioelectrochemistry, 2013, 94, 87–93 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |