Preparation of supported core–shell structured Pd@PdxSy/C catalysts for use in selective reductive alkylation reaction
Qunfeng Zhang,
Feng Feng,
Chang Su,
Wei Xu,
Lei Ma,
Chunshan Lu and
Xiaonian Li*
Institute of Industrial Catalysis, Zhejiang University of Technology, State Key Laboratory Breeding Base of Green Chemistry Synthesis Technology, Hangzhou, 310014, P. R. China. E-mail: xnli@zjut.edu.cn; Tel: +86-571-88320002
First published on 21st July 2015
Abstract
Supported noble-metal sulphide catalysts have attracted extensive scientific interest for their good selectivity in selective hydrogenation. However, the application of noble-metal catalysts is limited due to their lower activity, leading to harsh reaction conditions and poor conversion during hydrogenation reactions. In this study, Pd/C was sulphidized by H2S to prepare a series of core–shell structured Pd@PdxSy/C catalysts, which were characterized by BET, EDS, XPS, XRD and CO chemisorption to investigate the influences of sulphidation temperature, sulphidation time and sulphidation atmosphere on the structure of the resulting catalysts. The sulphidation of Pd/C at low temperatures resulted in a core–shell structured catalyst, Pd@PdxSy/C; with increasing sulphidation temperature, the size of Pd0 as the core decreased, and the thickness of palladium sulphides as the shell increased correspondingly. When the sulphidation temperature reached 150 °C, the resulting catalyst transformed to a complete palladium sulphide catalyst, PdxSy/C. The structure of Pd@PdxSy/C sulphidized at 30 °C was independent of sulphidation time and sulphidation atmosphere. The sulphidized catalysts were applied to the reductive alkylation of PADPA and MIBK to DBPPD. The sulphidized catalysts presented a much higher selectivity for DBPPD compared with Pd/C, and Pd@PdxSy/C showed higher activity than PdxSy/C; moreover, the greater amount of PdxSy content in the resulting catalyst led to a lower activity.
1 Introduction
Supported noble-metal catalysts are among the most industrially applied heterogeneous catalysts in various reductive reactions and have attracted extensive scientific interest.1–10 However, these catalysts are not qualified to catalyse the selective hydrogenation of organic compounds with multiple reducible functionalities because of their very high activity. Therefore, modifications of noble-metal catalysts for selective hydrogenation have been extensively applied in synthetic chemistry.5–10 This is also an effective way to improve the selectivity for the use of noble-metal sulphide catalysts during the selective hydrogenation.11–17 The supported noble-metal sulphide catalysts exhibit good performance in the reductive alkylation reaction of aldehydes/ketone and amine11–14 and the selective hydrogenation of halogenated nitrobenzene to the corresponding halogenated aniline.15–17 They are also suitable for the hydrogenation of sulfur-containing compounds.18–23 Moreover, the noble-metal sulphide catalysts, which are well known for the catalytic hydrotreatment, are widely used in hydrodesulfurization and hydrodenitrogenation.24–28However, the activity of the noble-metal sulphide catalysts is considerably lower than the unmodified noble-metal catalysts; thus, the noble-metal sulphide catalysts are often used under harsh conditions during hydrogenation.13–20 This severely limits their application. The key to solving this problem is improving the activity of noble-metal sulphide catalysts. Michele Breyssey et al. revealed that the activity of RuS2 catalysts depended on both the S/Ru and high S/Ru ratios, corresponding to low activity during their study about the catalytic mechanism of RuS2 catalysts.29,30 In our previous study, we also found that the catalytic activity of several types of activated carbon-supported palladium sulphides in the hydrogenation of 4-nitrothioanisole were ranked in the following order: Pd4S/C ≈ Pd16S7/C > Pd3S/C ≫ PdS/C,21 which indicated that Pd atoms with a smaller number of the coordinated S atoms showed relatively higher activity in the hydrogenation of sulfur-containing compounds. It appears to be an efficient way to improve the activity of noble-metal sulphide catalysts by reducing the number of coordinated S atoms.
Core–shell catalysts as the core particles coated with a functional material increase the physical properties such as electrical, optical, magnetic and thermal properties of the combined particles and exhibit unique catalytic performance.31–35 Recently, core–shell catalysts have been attracting increasing attention. Bao's group reported that Pt shells coated at the Cu core decorated with FeO patches used considerably less Pt but exhibited performance similar to that of only Pt nanoparticles covered with surface FeO patches in the catalytic oxidation of CO.34 Wei and co-workers prepared a polyaniline (PANI)-decorated Pt/C@PANI core–shell catalyst, and they found that this novel catalyst presented high activity and stability in the oxygen-reduction reaction for not only the electron delocalization between the Pt d orbitals and the PANI π-conjugated ligand but also electron transfer from Pt to PANI.35
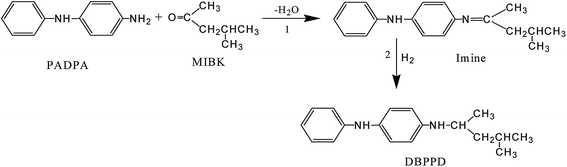
N-(1,3-Dimethylbutyl)-N′-phenyl-p-phenylenediamine (DBPPD) as a rubber antioxidant is synthesized by the reductive alkylation of p-aminodiphenylamine (PADPA) and methyl isobutyl ketone (MIBK) over industrial copper or noble-metal catalysts.36,37 In this reaction, PADPA and MIBK underwent a condensation reaction to form an imine compound, which hydrogenated in the presence of the catalyst to DBPPD (Scheme 1). PADPA, MIBK, imine and DBPPD are prone to undergo over-hydrogenation to byproducts using ordinary catalysts. Noble-metal sulphide catalysts, such as palladium sulphide and platinum sulphide, show high selectivity in the synthesis of DBPPD; however, their activity is insufficient.14
In this study, a series of activated carbon-supported Pd metal cores with PdxSy shell catalysts were prepared by a simple method and characterized by CO chemisorption, BET, EDS, XRD and XPS. The catalysts were then used in the reductive alkylation of PADPA and MIBK to DBPPD.
2 Experimental
2.1 Catalyst preparation
10% Pd/C was prepared by an incipient wetness impregnation method using H2PdCl4 as a Pd precursor. The commercially activated carbon (Xinsen Chemical Industry Co. Ltd) and deionized water were mixed in a weight ratio of 1 to 50. Then, a desired volume of 0.05 g ml−1 H2PdCl4 was added into the continuously stirred slurry solution at 80 °C, and the pH value of the sample was adjusted to 11 by adding 10% aqueous solution of NaOH. The precipitated Pd(OH)2 was reduced by hydrazine hydrate, which was then washed with deionized water until the pH value reached to about 7 and dried under vacuum at 110 °C for 10 h.A suspension of 1.0 g of 10% Pd/C and 50 ml deionized water was stirred in a three-necked flask, and a stream of gas containing H2S was then passed into the suspension under different conditions, as listed in Table 1. The resulting catalyst was collected on a filter paper, washed with deionized water until the pH was determined to be 7, and then dried under vacuum at 110 °C for 10 h.
| Catalyst | Sulphidation temperature/°C | Sulphidation atmosphere | Sulphidation time/h | Surface area/m2 g−1 | Mean diameter of Pd particles/nm | CO chemisorption uptake/ml g−1 | Elemental composition/wt% | Mole ratio of S/Pd | ||
|---|---|---|---|---|---|---|---|---|---|---|
| C | Pd | S | ||||||||
| SPC 1 | 30 | H2/H2S = 9/1 | 3 | 1508 | 5.9 | 0 | 81.79 | 9.73 | 1.45 | 0.50 |
| SPC 2 | 60 | H2/H2S = 9/1 | 3 | 1437 | 6.2 | 0 | 82.06 | 9.88 | 1.53 | 0.51 |
| SPC 3 | 100 | H2/H2S = 9/1 | 3 | 1552 | 6.5 | 0 | 82.61 | 9.82 | 1.55 | 0.52 |
| SPC 4 | 150 | H2/H2S = 9/1 | 3 | 1539 | 6.6 | 0 | 82.02 | 10.03 | 1.67 | 0.55 |
| SPC 5 | 200 | H2/H2S = 9/1 | 3 | 1477 | 7.0 | 0 | 82.31 | 9.68 | 1.72 | 0.59 |
| SPC 6 | 30 | H2/H2S = 9/1 | 5 | 1521 | 6.0 | 0 | 82.01 | 9.94 | 1.50 | 0.50 |
| SPC 7 | 30 | H2/H2S = 9/1 | 10 | 1570 | 6.1 | 0 | 81.33 | 9.67 | 1.49 | 0.51 |
| SPC 8 | 30 | N2/H2S = 9/1 | 3 | 1498 | 5.8 | 0 | 81.94 | 10.12 | 1.38 | 0.45 |
| SPC 9 | 30 | H2S | 3 | 1463 | 5.5 | 0 | 82.65 | 9.75 | 1.48 | 0.50 |
| SPC 10 | 30 | H2/H2S = 4/1 | 3 | 1509 | 5.8 | 0 | 82.58 | 9.84 | 1.48 | 0.50 |
| SPC 11 | 30 | H2/H2S = 49/1 | 3 | 1526 | 5.9 | 0 | 82.11 | 9.88 | 1.46 | 0.49 |
| Fresh Pd/C | — | — | — | 1517 | 5.7 | 3.5 | 82.53 | 9.85 | 0 | — |
2.2 Catalyst characterization
The morphologies and particle sizes of the catalysts were detected using a Philips-FEI Tecnai G2 F30 S-Twin transmission electron microscope operated at 300 kV.X-ray diffraction (XRD) of the catalysts was carried out on a Thermo ARL X'TRA diffractometer using Cu K-α radiation. The tube voltage was 40 kV and the tube current was 45 mA. The samples were scanned at 4° min−1.
X-ray photoelectron spectroscopy (XPS) analysis was performed with a Kratos AXIS Ultra DLD system. A monochromatized incident Al X-ray radiation (Al K-α = 1486.6 eV) with a fixed analyser pass energy of 80 eV was used for the excitation of the sample, and the binding energy values were referenced to the C 1s level (284.6 eV), resulting from surface contaminants.
The elemental content of the catalysts was detected using EDS (Thermo Vantage ESI).
The surface area of the catalysts was determined by nitrogen physical adsorption at 77 K. The samples were heated for 10 h to 473 K under vacuum conditions to remove the adsorbed species, and nitrogen adsorption isotherm was then obtained using a NOVA 1000e surface area analyzer (Quantachrome Instruments Corp.). The surface area of samples was calculated by the BET equation.
CO chemisorption uptake was measured by pulse chemisorption with a mass spectrometer (Omnistar TM) at ambient temperature and pressure.
2.3 Reductive alkylation reaction
Liquid-phase reductive alkylation was carried out at 200 °C and 3 MPa of hydrogen for 4 h in a 75 ml stainless-steel autoclave (Parr Instruments Company), which contained 3.7 g (0.02 mol) PADPA, 10 ml (0.08 mol) MIBK and 0.037 g catalyst. The final products were analyzed by a gas chromatograph (Agilent-6890) equipped with an HP-5 capillary column and an FID detector. The area normalization method was used for the quantitative determination of components.3 Results and discussion
3.1 Catalyst preparation and characterization
Supported metal sulphide catalysts are generally sulphidized from metal or metal oxide as the precursor by H2S or S as a sulphidation agent.14,38 The sulphidation conditions, such as sulphidation temperature, sulphidation time and sulphidation atmosphere, always cause important influences on the structure of the resulting supported metal sulphide catalysts.We first investigated the influence of sulphidation temperature on the structure of the palladium sulphide catalysts. 10 wt% Pd/C was sulphidized under an atmosphere of H2/H2S with a volume ratio of 9/1 for 3 h at the temperatures of 30, 60, 100, 150, and 200 °C, respectively, and the resulting catalysts, denoted as SPC 1–5, were characterized using N2 physisorption for surface area, CO chemisorption, TEM, EDS, XPS, and XRD. The surface areas of SPC 1–5 (Table 1) were almost the same as that of fresh Pd/C, suggesting that the influence of the sulphidation temperature on the activated carbon structure was negligible. The morphology of Pd particles of fresh Pd/C and SPC 1–5 observed by TEM (Fig. 1) showed that the Pd particle sizes of the six catalysts were about 4–10 nm. The elemental content of SPC 1–5 catalysts, determined by EDS (Table 1), showed that all the five catalysts after sulphidation contained S, and its content increased from 1.45 wt% to 1.76 wt% with the sulphidation temperature from 30 °C to 200 °C, whereas the content of Pd in SPC 1–5 was nearly unchanged. CO chemisorption is used to determine the content of Pd0 on the surface of the supported palladium catalyst. In our experiment, the CO chemisorption uptakes of five catalysts (Table 1) after sulphidation at various temperatures were 0 ml g−1, whereas the CO chemisorption uptake of the fresh Pd/C was 3.5 ml g−1. This result indicates that there was no Pd0 on the surface of the catalysts after sulphidation at different temperatures. Because the content of Pd in SPC 1–5 did not decrease after sulphidation, we can infer that the Pd metal on the surface of these catalysts had been converted to palladium sulphide.
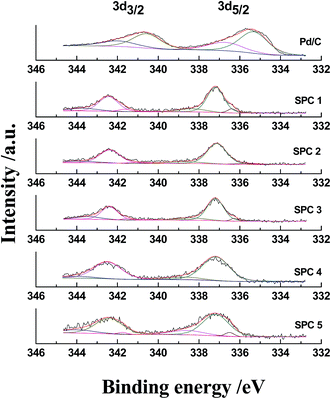
The fresh Pd/C and SPC 1–5 catalysts were also investigated by XPS (Fig. 2 and Table 2). The fitted XPS spectra of Pd 3d5/2 for the fresh Pd/C exhibit peaks at 335.3 eV and 336.7 eV, which can be attributed to Pd0 and Pd2+, respectively, because a part of palladium atoms on the surface of the Pd/C catalyst was oxidized by air. However, no peak was observed at about 335.3 eV for the catalysts SPC 1–5, which suggests that there was no Pd0 on the surface of the SPC 1–5 catalysts. The fitted XPS spectra of Pd 3d5/2 of SPC 1–5 contain peaks at 336.4, 337.1 and 338.2 eV, meaning that all the Pd atoms on the surfaces of these catalysts had been changed to high-valency palladium ions as palladium sulphides. This is consistent with the results of CO chemisorption examinations. There are several types of palladium sulphide crystalline phases, such as Pd4S, Pd3S, Pd16S7, PdS and PdS2, with different valencies of Pd elements. It is also found that the areas of the peaks at 337.1 and 338.2 eV increase with increasing sulphidation temperature, which indicates that there were more high-valency palladium ions in the catalyst sulphidized at higher temperatures. The XRD patterns of the fresh Pd/C and sulphidized catalysts are shown in Fig. 3. The broad diffraction peaks at about 2θ = 25° and 44° are ascribed to the activated carbon. The sharp diffraction peaks of Pd for the fresh Pd/C appear at 2θ = 40.1°, 46.6° and 68.1°. The XRD pattern of the catalyst SPC 1 sulphidized at 30 °C is similar to that of the fresh Pd/C, and no peaks of palladium sulphides are observed, indicating that the catalyst sulphidized at 30 °C did not form a palladium sulphide crystal, and that most of the palladium on the sulphidized catalyst still existed as Pd0. The results of CO chemisorption and XPS as previously described have confirmed that Pd atoms on the surface of the sulphidized catalyst had all been changed to palladium sulphides, and these palladium sulphides located just on the surface of the palladium particles and the core of palladium particles should be still Pd0. Therefore, the catalyst SPC 1 exhibits a structure with Pd0 as the core and the membrane of palladium sulphides as the shell (Pd@PdxSy/C). With increasing sulphidation temperature, the peaks of Pd0 for the sulphidized catalysts become weak and broad. The palladium particle size observed from TEM did not change significantly after sulphidation, which means that the particle sizes of Pd0 for the resulting catalysts was decreased with increasing sulphidation temperature. When the sulphidation temperature was increased to 150 °C, the Pd0 diffraction peaks disappeared, which suggests that the Pd0 particle on this catalyst has been reduced to a range undetectable by XRD. We also observe that the diffraction peaks of Pd4S appear on the XRD pattern of the catalyst sulphidized at 150 °C, and the intensity of the Pd4S peaks is further enhanced when the sulphidation temperature is increased to 200 °C. This indicates that the content of Pd4S was increased, and the particle of Pd4S grew larger with an increase in sulphidation temperature. Therefore, the sulphidation temperature has a significant influence on the structures of the resulting catalysts. When Pd/C was sulphidized at a low temperature, the sulphidized catalyst was a core–shell structure, Pd@PdxSy/C. By increasing the sulphidation temperature, the size of Pd0 as the core decreased, and the thickness of palladium sulphides as the shell increased correspondingly. Moreover, when the sulphidation temperature was increased to 150 °C, the resulting catalyst transformed to a complete palladium sulphide catalyst, PdxSy/C.
| Catalyst | Peak 1 | Peak 2 | Peak 3 | |||
|---|---|---|---|---|---|---|
| Binding energy/eV | Peak area/% | Binding energy/eV | Peak area/% | Binding energy/eV | Peak area/% | |
| SPC 1 | 336.4 | 6.6 | 337.2 | 78.1 | 338.3 | 15.3 |
| SPC 2 | 335.8 | 3.4 | 337.1 | 83.6 | 338.7 | 13.0 |
| SPC 3 | 336.3 | 4.4 | 337.2 | 81.9 | 338.3 | 13.7 |
| SPC 4 | — | — | 337.2 | 88.4 | 338.7 | 11.6 |
| SPC 5 | 336.5 | 4.8 | 337.2 | 75.5 | 338.6 | 19.7 |
| SPC 6 | 336.3 | 4.7 | 337.2 | 83.6 | 338.3 | 11.7 |
| SPC 7 | 336.2 | 3.9 | 336.9 | 85.2 | 338.5 | 10.9 |
| SPC 8 | 335.8 | 6.3 | 337.0 | 84.8 | 338.3 | 8.9 |
| SPC 9 | 335.8 | 4.1 | 336.7 | 88.1 | 338.2 | 7.8 |
| SPC 10 | 335.8 | 5.0 | 337.0 | 83.8 | 338.2 | 11.2 |
| SPC 11 | 336.0 | 10.8 | 336.8 | 77.5 | 338.1 | 11.7 |
| Fresh Pd/C | — | — | 335.3 | 71.7 | 336.7 | 28.3 |
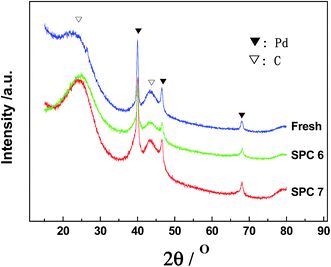
The influence of sulphidation time on Pd@PdxSy/C catalysts was also investigated. 10 wt% Pd/C was sulphidized under an H2/H2S atmosphere with the volume ratio of 9/1 at 30 °C for 5 and 10 h, respectively, and the resulting catalysts were designated as SPC 6 and 7. The surface areas of this series of sulphidized catalysts ranged from 1508 to 1570 m2 g−1, similar to that of fresh Pd/C; this implies that the sulphidation time also had a little impact on the structure of the activated carbon. The TEM images (Fig. 1) also showed that the size of the Pd particles did not change significantly after sulphidation treatment for different times. The content of S in SPC 1, 6, and 7 detected by EDS were maintained in the range of 1.45–1.50 wt%, whereas the Pd content in these catalysts were maintained in the range of 9.67–9.94 wt%. The CO chemisorption uptakes for all three catalysts were at 0 ml g−1. SPC 6 and 7 were also investigated using XPS and XRD. The XPS Pd 3d5/2 spectra of these catalysts (Fig. 4 and Table 2) exhibited peaks at 336.4, 337.1 and 338.2 eV, and there no peak was observed at 335.3 eV. The results of CO chemisorption and XPS show that all the Pd atoms on the surfaces of the catalysts had been transformed to palladium sulphides. XRD patterns of SPC 6 and 7 (Fig. 5) exhibited only the diffraction peaks of Pd0 and activated carbon, and no peaks for palladium sulphides were detected. Furthermore, the intensity of the Pd0 peaks is similar to that of the fresh Pd/C. Therefore, after the sulphidation for 3, 5 and 10 h, the resulting catalysts still maintained Pd0 as the core. In addition, the particle size of the Pd0 core did not unambiguously decrease. All the catalysts sulphidized for different times were core–shell, Pd@PdxSy/C; moreover, the structure of the Pd@PdxSy/C catalysts is almost independent of sulphidation time.
Recently, we reported that the formation of palladium sulphides supported on the activated carbon is highly dependent on the composition of sulphidation atmosphere.14 We then investigated the influence of the sulphidation atmosphere on the structure of the PdxSy@Pd/C catalysts. 10 wt% Pd/C was sulphidized for 3 h at 30 °C under N2/H2S with the volume ratio of 9/1, in addition to pure H2S and the H2/H2S atmosphere with volume ratios of 4/1 and 49/1, respectively, and the resulting catalysts were denoted as SPC 8–11. The surface areas of these catalysts were similar to that of fresh Pd/C. The content of S in SPC 11 sulphidized under the atmosphere of H2/H2S with a volume ratio of 49/1 was only 1.38 wt%, which was considerably lower than that of other sulphidized catalyst. This should be caused by the low content of H2S in the atmosphere with a sulphidation time of only 3 hours, leading to incomplete sulphidation. When the content of H2S in the atmosphere was increased to 4/1, and even when it was used as pure H2S, the S content in the resulting catalyst increased negligibly. The same result was also found during the sulphidation of Pd/C with N2/H2S with a volume ratio of 9/1. The CO chemisorption uptakes of SPC 8–11 were all 0 ml g−1, which indicated that all the five catalysts, including the incompletely sulphidized SPC 11, had no Pd0 on their surface. This result was also confirmed by the XPS spectra (Fig. 6 and Table 2) of these catalysts for their fitted Pd 3d5/2 peaks only at 336.4, 337.1 and 338.2 eV, which meant that there were only high-valence palladium ions on the surface. Moreover, the area of peak at 336.4 eV for SPC 11 is considerably larger than that of other catalysts, which should be due to incomplete sulphidation. The XRD patterns of SPC 8–11 (Fig. 7) are similar to that of fresh Pd/C, and no palladium sulphide peaks are observed, suggesting that there was only Pd metal crystal as the core. Therefore, Pd/C sulphidized under different atmospheres also resulted in a core–shell catalyst, Pd@PdxSy/C, and the influence of the sulphidation atmosphere was also not significant.
The sulphidation process of Pd/C with H2S to Pd@PdxSy/C and PdxSy/C is shown in Fig. 8. First, H2S was adsorbed on the surface of Pd atoms, and then H2S was cracked and reacted with Pd to yield palladium sulphides, and H2 was released simultaneously. Therefore, the Pd atoms on the surface of Pd/C were first sulphidized to palladium sulphides during the sulphidation process. According to the previously discussed results, we conclude that certain conditions such as high sulphidation temperature to sulphidize the Pd metal in the core of Pd particle to palladium sulphide were required. If the temperature was very low (for example, room temperature) the use of a simple method of increasing sulphidation time or changing sulphidation atmosphere was not able to transform all the Pd atoms to palladium sulphides, and just sulphidized the Pd atoms on the surface to form a core–shell catalyst, Pd@PdxSy/C. The reason should be that the adsorption and cracking of H2S became more difficult on palladium sulphides than on Pd metal, and obtaining a sufficient amount of sulfur from the palladium atoms in the core of Pd@PdxSy to generate palladium sulphide was highly difficult. The thickness of PdxSy on Pd@PdxSy/C was dependent on the sulphidation temperature. After increasing the sulphidation temperature, H2S was promoted to be cracked on the surface of the catalyst, and the shell of PdxSy was then thickened. When the sulphidation temperature was increased to 150 °C, it was sufficient to completely sulphidize the entire Pd particle into palladium sulphide. Therefore, SPC 4 and SPC 5 had been completely sulphidized to PdxSy/C, and the other sulphidized catalysts formed a core–shell structure, Pd@PdxSy/C.
3.2 Catalytic performances
The catalytic performances of Pd@PdxSy/C, PdxSy/C and Pd/C were tested for the reductive alkylation of MIBK and PADPD to DBPPD under the reaction conditions of 200 °C and 3 MPa for 4 h (Scheme 1), and the results are listed in Table 3. 100% conversion of PADPD was obtained over Pd/C catalyst, but most of PADPD was converted to by-products such as N-(1,3-dimethylbutyl)-N′-cyclohexane-p-phenylenediamine and aniline, and the selectivity of DBPPD was only 39.5%. In contrast, with the exception of SPC 11, all the sulphidized catalysts presented a considerably higher selectivity of DBPPD of up to 96.7%. The low selectivity for SPC 11 in the reductive alkylation should be caused by its incomplete sulphidation under the atmosphere of H2/H2S with a volume ratio of 49/1, leading to its content of PdxSy on the surface being lower than that of other sulphidized catalysts. The sulphidized catalysts presented a lower activity than Pd/C for reductive alkylation. However, Pd@PdxSy/C showed higher activity than PdxSy/C for the conversions of PADPD for SPC 4 and SPC 5 at only 93.2% and 90.3%, whereas the conversions of PADDP for all the other sulphidized catalysts were more than 98.3%. In addition, we also observed that the activity of the sulphidized catalyst decreased with increasing sulphidation temperature, which meant that more content of PdxSy in the resulting catalyst led to lower activity.| Catalyst | Conversion/% | Selectivity/% | ||
|---|---|---|---|---|
| DBPPD | Imine | Others | ||
| SPC 1 | 100 | 98.2 | 0.2 | 1.6 |
| SPC 2 | 99.4 | 97.5 | 0.4 | 2.1 |
| SPC 3 | 98.3 | 97.4 | 1.1 | 1.5 |
| SPC 4 | 93.2 | 97.0 | 1.5 | 1.5 |
| SPC 5 | 90.3 | 96.7 | 1.9 | 1.4 |
| SPC 6 | 99.9 | 97.9 | 0.1 | 2.0 |
| SPC 7 | 99.8 | 97.9 | 0.2 | 1.9 |
| SPC 8 | 100 | 97.9 | 0.1 | 2.0 |
| SPC 9 | 99.8 | 97.6 | 0.3 | 2.1 |
| SPC 10 | 99.8 | 97.5 | 0.2 | 2.3 |
| SPC 11 | 100 | 90.1 | 0 | 9.9 |
| Pd/C | 100 | 39.5 | 0 | 60.5 |
Researchers29,30 had investigated the catalytic hydrogenation mechanism of RuS2 catalyst, and they found that H2 adsorbed on the surface of RuS2 due to homolytic adsorption of dihydrogen and heterolytic dissociation on an Ru–S site, leading to Ru–H and SH groups. In addition, the activity was directly proportional to the amount of homolytic adsorption of dihydrogen, which adsorbed on the Ru centers in low-sulfur coordination. The mechanism for palladium sulphide catalyst should be similar to that of RuS2 catalyst. Pd@PdxSy/C is a catalyst with a membrane of palladium sulphide as the shell, thus it has high selectivity as a common PdxSy/C catalyst during selective hydrogenation. In addition, its core of palladium metal causes the Pd ions on the surface to exhibit a lower sulfur coordination than that of common PdxSy/C catalyst, which improves the activity. Thus, Pd@PdxSy/C is a hydrogenation catalyst with a combination of high selectivity and good activity.
4 Conclusion
A simple and effective method for the preparation of a core–shell Pd@PdxSy/C catalyst through sulphidation with H2S is proposed. The sulphidation temperature played an important role in the structure of the sulphidized catalyst. The low sulphidation temperature resulted in a core–shell structured catalyst, Pd@PdxSy/C. With increasing sulphidation temperature, the size of Pd0 as the core decreased, and the thickness of palladium sulphides as the shell increased correspondingly. Moreover, when the sulphidation temperature reached 150 °C, the resulting catalyst transformed to a complete palladium sulphide catalyst, PdxSy/C. The structure of Pd@PdxSy/C sulphidized at 30 °C was independent of sulphidation time and sulphidation atmosphere. The sulphidized catalysts presented considerably higher selectivity for DBPPD, compared with Pd/C during the reductive alkylation of PADPA and MIBK to DBPPD, and Pd@PdxSy/C showed slightly higher activity than PdxSy/C. Pd@PdxSy/C is a promising catalyst with a combination of high selectivity and good activity for selective hydrogenation reactions.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (NSFC-21406199 and 21476208) and the program from the Science and Technology Department of Zhejiang Province (2015C31042).Notes and references
- J. Lu, B. Fu, M. C. Kung, G. Xiao, J. W. Elam, H. H. Kung and P. C. Stair, Science, 2012, 335, 1205–1208 CrossRef CAS PubMed.
- J. Zhao, T. Zhang, X. Di, J. Xu, J. Xu, F. Feng, J. Ni and X. Li, RSC Adv., 2015, 5, 6925–6931 RSC.
- J. A. Anderson, A. Athawale, F. E. Imrie, F.-M. McKenna, A. McCue, D. Molyneux, K. Power, M. Shand and R. P. K. Wells, J. Catal., 2010, 270, 9–15 CrossRef CAS PubMed.
- T. Xu, Q. Zhang, D. Jiang, Q. Liang, C. Lu, J. Cen and X. Li, RSC Adv., 2014, 4, 33347–33354 RSC.
- Y. Chen, X. Zhang and S. Mitra, Chem. Commun., 2010, 46, 1652–1654 RSC.
- Q. Zhang, L. Ma, C. Lu, X. Xu, J. Lyu and X. Li, React. Kinet., Mech. Catal., 2015, 114, 629–638 CrossRef CAS.
- Y. Wang, X. Cui, Y. Deng and F. Shi, RSC Adv., 2014, 4, 2729–2732 RSC.
- C. Lian, H. Liu, C. Xiao, W. Yang, K. Zhang, Y. Liu and Y. Wang, Chem. Commun., 2012, 48, 3124–3126 RSC.
- Q. Zhang, C. Su, J. Cen, F. Feng, L. Ma, C. Lu and X. Li, Chin. J. Chem. Eng., 2014, 22, 1111–1116 CrossRef CAS PubMed.
- A. J. McCue and J. A. Anderson, Catal. Sci. Technol., 2014, 4, 272–294 CAS.
- F. S. Dovell and H. Greenfield, J. Org. Chem., 1964, 29, 1265–1267 CrossRef CAS.
- H. Greenfield and F. S. Dovell, J. Org. Chem., 1966, 31, 3053 CrossRef CAS.
- Q. Zhang, J. Wu, C. Su, F. Feng, Q. Ding, Z. Yuan, H. Wang, L. Ma, C. Lu and X. Li, Chin. Chem. Lett., 2012, 23, 1111–1114 CrossRef CAS PubMed.
- W. Xu, J. Ni, Q. Zhang, F. Feng, Y. Xiang and X. Li, J. Mater. Chem. A, 2013, 1, 12811–12817 CAS.
- H. Greenfield and F. S. Dovell, J. Org. Chem., 1967, 32, 3670–3671 CrossRef CAS.
- F. S. Dovell and H. Greenfield, J. Am. Chem. Soc., 1965, 87, 2767–2768 CrossRef CAS.
- J. Frimmel and M. Zdrazil, J. Catal., 1997, 167, 286–295 CrossRef CAS.
- A. V. Mashkina and L. G. Sakhaltueva, Kinet. Catal., 2002, 43, 107–114 CrossRef CAS.
- A. V. Mashkina and A. A. Zirka, Kinet. Catal., 2000, 41, 521–526 CrossRef CAS.
- A. V. Mashkina and T. S. Sukhareva, React. Kinet. Catal. Lett., 1999, 67, 103–110 CrossRef CAS.
- Q. Zhang, W. Xu, X. Li, D. Jiang, Y. Xiang, J. Wang, J. Cen, S. Romano and J. Ni, Appl. Catal., A, 2015, 497, 17–21 CrossRef CAS PubMed.
- C. Sun, M.-J. Peltre, M. Briend, J. Blanchard, K. Fajerwerg, J.-M. Krafft, M. Breysse, M. Cattenot and M. Lacroix, Appl. Catal., A, 2003, 245, 245–255 CrossRef CAS.
- M. Breysse, M. Cattenot, V. Kougionas, J. C. Lavalley, F. Mauge, J. L. Portefaix and J. L. Zotin, J. Catal., 1997, 168, 143–153 CrossRef CAS.
- N. Hermann, M. Brorson and H. Topsøe, Catal. Lett., 2000, 65, 169–174 CrossRef CAS.
- J. A. D. L. Reyes, Appl. Catal., A, 2007, 322, 106–112 CrossRef PubMed.
- Y. Aray and J. Rodríguez, ChemPhysChem, 2001, 2, 599–604 CrossRef CAS.
- S. Eijsbouts, V. H. J. D. Beer and R. Prins, J. Catal., 1988, 109, 217–220 CrossRef CAS.
- M. Lacroix, N. Boutarfa, C. Guillard, M. Vrinat and M. Breysse, J. Catal., 1989, 120, 473–477 CrossRef CAS.
- C. Dumonteil, M. Lacroix, C. Geantet, H. Jobic and M. Breyssey, J. Catal., 1999, 187, 464–473 CrossRef CAS.
- B. Moraweck, G. Bergeret, M. Cattenot, V. Kougionas, C. Geantet, J.-L. Portefaix, J. L. Zotin and M. Breysse, J. Catal., 1997, 165, 45–56 CrossRef CAS.
- R. G. Chaudhuri and S. Paria, Chem. Rev., 2012, 112, 2373–2433 CrossRef PubMed.
- L. Lin, T. Zhang, X. Zhang, H. Liu, K. L. Yeung and J. Qiu, Ind. Eng. Chem. Res., 2014, 53, 10906–10913 CrossRef CAS.
- Y. Wei, Z. Zhao, J. Liu, S. Liu, C. Xu, A. Duan and G. Jiang, J. Catal., 2014, 317, 62–74 CrossRef CAS PubMed.
- X. Guo, Q. Fu, Y. Ning, M. Wei, M. Li, S. Zhang, Z. Jiang and X. Bao, J. Am. Chem. Soc., 2012, 134, 12350–12353 CrossRef CAS PubMed.
- S. Chen, Z. Wei, X. Qi, L. Dong, Y. Guo, L. Wan, Z. Shao and L. Li, J. Am. Chem. Soc., 2012, 134, 13252–13255 CrossRef CAS PubMed.
- H. L. Merten and L. M. Baclawski, US Pat., 4900868, 1990.
- R. M. D'Sidocky and D. K. Parker, US Pat., 4463191, 1984.
- A. Zubkov, T. Fujino, N. Sato and K. Yamada, J. Chem. Thermodyn., 1998, 30, 571–581 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |