An acid functionalized MWCNT/PVP nanocomposite as a new additive for fabrication of an ultrafiltration membrane with improved anti-fouling resistance
Abstract
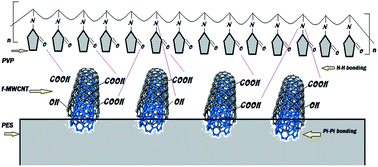
Membrane fouling is one of the main challenges encountered in ultrafiltration (UF) processes and the use of nanoparticles for the improvement of UF performance is a recent trend in membrane technology. In this study, in order to improve surface characteristics of polyethersulfone (PES)-based membranes for greater resistance against biofouling, PES was incorporated with a new type of nanocomposite (NC) in which the NC could be synthesized by blending acid functionalized multiwalled carbon nanotubes (f-MWCNT) with polyvinylpyrrolidone (PVP) in dimethylformamide (DMF). The chemistry of the NCs embedded within the PES membrane matrix was analysed by FTIR, whereas the fabricated membranes were characterized by FESEM, contact angle, water absorption tests, surface profile studies and their filtration performances with respect to pure water permeation, antifouling resistance against proteins and flux recovery rate. The results revealed that, compared to the pristine PES membrane, the antifouling ability of the PES membrane incorporated with f-MWCNT/PVP NC is greater, recording 81.7% flux recovery and 80.2% total resistance (>76% were reversible one). The protein separation results indicated that, the NCs based membrane was able to reject 93.4%, 74.7%, 59.4% and 28.5% for bovine serum albumin (66 kDa), pepsin (34.6 kDa), trypsin (20 kDa) and (14.6 kDa), respectively.

 Please wait while we load your content...
Please wait while we load your content...