Retracted Article: Shape-specific silver nanoparticles prepared by microwave-assisted green synthesis using pomegranate juice for bacterial inactivation and removal†
Ekta Roya,
Santanu Patraa,
Shubham Sahaa,
Rashmi Madhuri*a and
Prashant K. Sharmab
aDepartment of Applied Chemistry, Indian School of Mines, Dhanbad, Jharkhand 826 004, India. E-mail: rshmmadhuri@gmail.com; Tel: +91 9471191640
bFunctional Nanomaterials Research Laboratory, Department of Applied Physics, Indian School of Mines, Dhanbad, Jharkhand 826 004, India
First published on 16th October 2015
Abstract
Herein, we have synthesized four different shapes (spherical, oval, rod and flower shape) of silver nanoparticles (AgNPs) using a green synthesis approach via microwave-assisted synthesis. The nanoparticles were synthesized using pomegranate juice as a novel reducing agent. The synthesized AgNPs were characterized by UV-vis spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM) and tunnelling electron microscopic (TEM) analysis. The as-prepared AgNPs show a very rapid, effective, shape-specific and dose-dependent bacteriostatic/bactericidal effect towards four different bacterial strains (two Gram negative and two Gram positive). The analysis was determined by different methods such as a disc diffusion study, growth curve analysis, minimum inhibitory concentration and minimum bactericidal concentration determinations. Amongst the four different shaped AgNPs, the flower shaped AgNPs demonstrated the best results and mediated the fastest bactericidal activity against all the tested strains at similar bacterial concentrations. The results were also confirmed by confocal microscopic analysis. Additionally, the flower shaped AgNPs have the ability to non-specifically remove various bacterial strains from a real water sample with ∼98% removal efficiency, as compared to other synthesized different shaped nanoparticles.
Introduction
A big challenge in the field of pharmaceuticals and biomedicines is resistant of human beings towards pathogens or bacteria's. In this, the anti-biotic resistance has acquired a large range of fear for emergence and re-emergence of multidrug-resistant (MDR) pathogens and parasites.1,2 When a person gets infected with MDR bacteria, their cure becomes very difficult, resulting in loss of money as well as time in hospitals. The MDR bacteria infected patient requires multiple treatments of broad-spectrum antibiotics, which are less effective, more toxic and more expensive.3,4 Therefore, some modifications and development in anti-bacterial compounds with improved bacteriostatic and bactericidal effects are now a high priority in the field of research.5,6From past decades, silver has been used as an antimicrobial compound to deal with infections, burns and chronic wounds due to its low toxicity to the human body. Many scientists have reported that silver nanoparticles (AgNPs) with a size in the range of 10–100 nm showed strong bactericidal potential against both Gram-positive and Gram-negative bacteria.7 Though the mechanism of antibacterial killing or prevention is not very clear to date, some of the authors have discussed the mechanism based on their own obtained results. Accordingly, AgNPs may penetrate the bacterial cell wall and modulate cellular signaling by dephosphorylating peptide substrates on tyrosine residues.8–10 During the study, it was also found that the antibacterial property of AgNPs depends up on the size, morphology, stability and (chemical and physical) properties of the nanoparticles. The shape, size and other factors are strongly influenced by the experimental conditions viz., kinetics of the interaction of metal ions with reducing agents, and adsorption processes of stabilizing agents with metal nanoparticles.11 Generally, specific control of the shape, size and distribution of the produced nanoparticles could be achieved by changing the methods of synthesis, the reducing agents and stabilizers.12–15
During the last three decades, synthesis of metal nanoparticles using natural resources i.e. green technology and/or synthesis has been increased a lot. Using natural precursors or natural compounds as intermediate chemicals in nanoparticle synthesis is not only an environmental friendly step, it is a step towards less-harmful and more cost-effective chemical synthesis.16 In the literature, several works have been reported for the synthesis of AgNPs using a green synthesis approach. Vinod et al. have reported the synthesis of Ag, Au and Pt nanoparticles using a natural hydrocolloid gum kondagogu (Cochlospermum gossypium) as a precursor molecule.17 Logeswari et al. reported the synthesis of AgNPs from commercially available plant powders i.e. Solanum trilobatum, Syzygium cumini, Centella asiatica and Citrus sinensis.18 AgNPs have also been synthesised from three medicinal leaf extracts, Musa balbisiana (banana), Azadirachta indica (neem) and Ocimum tenuiflorum (black tulsi), by Banerjee et al.19
Although, several studies have been reported the synthesis of AgNPs using chemical routes and their applications in antibacterial studies, the synthesis of shape-specific nanoparticles using a green synthesis approach is rarely reported. The effect of shape specific nanoparticles on bacterial growth was first explored by Pal et al.,20 where three different shaped AgNPs (spherical, rod shaped and truncated) were reported. It was found that the truncated AgNPs had a better response for antibacterial purposes (Sodium borohydride). Similarly, Dong et al. have reported the synthesis of triangular nanoprism and spherical AgNPs and it was reported that the triangular-shape nanoparticle had a better antibacterial property than the spherical one due to its geometric structure and geometric plane.21 To the best of our knowledge, a green chemistry approach for the synthesis of shape-specific AgNPs as antimicrobial agents has not been reported yet.
The present work has focused on the development of an easy and one-pot synthesis of AgNPs by an environmentally friendly procedure (microwave assisted method) with pomegranate fruit juice as the reducing agent. Microwave irradiation has several advantages in comparison to the traditional heating methods. It produced uniform heating of the solution (short thermal induction period) and as a result, more homogeneous nucleation (less-time) with a short crystallization time is obtained at a much lower cost.22 In addition, herein, pomegranate fruit juice was used as a reducing agent which contains mainly gallic acid, ellagic acid and flavonol glycosides that are able to reduce silver nitrate into AgNPs very efficiently.23 The nanoparticles were prepared as four different shapes, namely, spherical, oval, rod and flower. The synthesized AgNPs have been characterized by field emission scanning electronic microscopy (FE-SEM), transmission electron microscopy (TEM), UV-visible spectroscopy and powder X-ray diffraction (XRD) techniques. The bactericidal activity of the as prepared different shaped AgNPs against the pathogenic, MDR as well as multi-drug susceptible strains of bacteria such as Pseudomonas aeruginosa (Gram negative), ampicillin-resistant Escherichia coli (Gram negative), Bacillus subtilis (Gram positive), and methicillin-resistant Staphylococcus aureus (Gram positive) were studied. It was found that the shape of the nanoparticle plays a very important role in the killing of the bacterial strain. In addition, some real water samples were also used to explore the water purification impact of the synthesized nanoparticles.
Experimental section
Reagents and instruments
Silver nitrate (AgNO3) and cetyltrimethylammonium bromide (CTAB) were purchased from Fluka. All chemicals and reagent used here were of analytical grade or higher.The synthesized nanoparticles were characterized by Field emission scanning electron microscope (FE-SEM, Zeiss, model Super 55). The powder X-ray diffraction (XRD) study was carried out on a Bruker D8 Focus X-ray diffractometer instrument using a Cu target radiation source. Transmission electron microscopy (TEM) studies were performed on a Tecnai 30 G2 S-Twin electron microscope operated at 300 kV accelerating voltage. UV-visible spectroscopic characterization was done on a Perkin-Elmer Lambda 35 (Singapore) spectrophotometer.
Preparation of the pomegranate extract
The pomegranate was collected from a local market on the basis of cost effectiveness and ease of availability. Fresh and healthy pomegranate were collected and rinsed thoroughly first with tap water followed by distilled water to remove all the dust and unwanted visible particles. Seeds were separated from the fruit, crushed and juice was collected in a beaker (250 mL). To this, 100 mL distilled water was added and the mixture was boiled for about 20 min. After that, the juice was filtered and stored at 4 °C, before use.Synthesis of the different shaped silver nanoparticles (AgNPs)
For the synthesis of the different shaped AgNPs, AgNO3 was used as the precursor compound with CTAB as the stabilizer and pomegranate juice as the reducing agent. The amount of constituents and reaction conditions were varied to synthesize the different shaped AgNPs. The procedures are given below and shown in Scheme 1.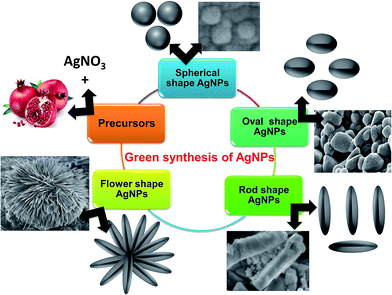 | ||
| Scheme 1 Graphical representation for synthesis of shape-specific silver nanoparticle using green synthesis approach. | ||
Preparation of bacterial samples and antibacterial study
The various shaped AgNPs synthesized using pomegranate juice were applied to explore their shape-specific properties towards different bacterial strains i.e. Pseudomonas aeruginosa (Gram negative), Escherichia coli (Gram negative), Bacillus subtilis (Gram positive), and Staphylococcus aureus (Gram positive). The study was performed by different analysis methods: (1) a disc diffusion method, (2) minimum inhibition concentration (MIC), (3) minimum bactericidal concentration (MBC) and (4) growth kinetics.| Death rate (%) = [(counts in the control) − (counts upon incubation with the nanoparticle)]/(counts in the control) | (1) |
Removal of bacteria by flower shaped AgNPs
Nowadays, the water purification system including filtration membranes etc. are modified with nanoparticles to remove bacterial contaminations more efficiently. Herein, we have also tried to explore the water purification aspect of the flower shaped AgNPs for removal of bacterial contamination from water samples collected from different areas of our state. For the analysis, the diluted bacterial solution (1 × 103 CFU mL−1) was added in a glass vial containing 10.0 mL of F-AgNPs (20.0 μg L−1). The mixed solution was placed in a rotary shaker for 15 min to ensure the effective binding between the bacteria and nanoparticles. After that, the nanoparticles were filtered and separated from the mixture solution and the remaining supernatant was used to analyze the bacterial count by a conventional surface plate count method. The bacteria removal efficiency of the F-AgNPs was calculated using eqn (2):| Removal efficiency (%) = (CFU0 − CFUt)/CFU0 × 100 | (2) |
Result and discussion
Characterization of AgNPs
Antibacterial study
| S-AgNPs < O-AgNPs < R-AgNPs < F-AgNPs |
| Concentration (μg L−1) | Gram negative bacterial strain | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | |||||||||||||||||||
| S-AgNPs | O-AgNPs | R-AgNPs | F-AgNPs | AgNO3 | S-AgNPs | O-AgNPs | R-AgNPs | F-AgNPs | AgNO3 | |||||||||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| 25.0 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 20.0 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 15.0 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 12.0 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 10.0 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 8.0 | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | + |
| 6.0 | − | − | − | − | − | − | − | − | + | + | − | − | − | − | − | − | − | − | + | + |
| 3.0 | − | + | − | − | − | − | − | − | + | + | − | + | − | − | − | − | − | − | + | + |
| 2.0 | + | + | − | + | − | − | − | − | + | + | + | + | − | + | − | − | − | − | + | + |
| 1.0 | + | + | + | + | − | + | − | − | + | + | + | + | + | + | − | + | − | − | + | + |
| 0.60 | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | − | + | + | + |
| 0.50 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.40 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.30 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.10 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.05 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Concentration (μg L−1) | Gram positive bacterial strain | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. subtilis | S. aureus | |||||||||||||||||||
| 25.0 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 20.0 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 15.0 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 12.0 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| 10.0 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + |
| 8.0 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + |
| 6.0 | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | + | + |
| 3.0 | − | − | − | − | − | − | − | − | − | + | + | + | − | + | − | − | − | − | + | + |
| 2.0 | − | − | − | − | − | − | − | − | − | + | + | + | + | + | − | + | − | − | + | + |
| 1.0 | − | − | − | − | − | − | − | − | − | + | + | + | + | + | + | + | − | + | + | + |
| 0.60 | − | + | − | − | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.50 | + | + | − | − | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.40 | + | + | − | + | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.30 | + | + | + | + | − | + | − | − | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.10 | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 0.05 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
Similarly, for the Gram positive bacterial strains (B. subtilis and S. aureus), the lowest MIC values were found for F-AgNPs, 0.1 μg L−1 and 1.0 μg L−1, respectively, and an increase in the value was obtained with same trend as presented for Gram negative bacteria i.e. F-AgNPs < R-AgNPs < O-AgNPs < S-AgNPs (Table 1). Therefore, it is clearly demonstrated that the shape of nanoparticles plays a very important role on their antibacterial activity and herein, the multi-plane structure i.e. F-AgNPs shows the maximum response towards all the four bacterial strains.
As discussed in the UV and SEM analysis, during the structural transformation, the rod-shaped or flower-shaped AgNPs may contain some amount of oval or spherical nanoparticles. Their separation is not possible. Therefore, to study their coordinated effect on the antibacterial activity, we have conducted a MIC study with different mixtures of AgNPs. The corresponding data are portrayed in Table S1.† As shown in the table, a mixture containing the same concentration of S-AgNPs and F-AgNPs was unable to inhibit the growth of bacteria (1.0 μg L−1 for Gram negative and 0.1 μg L−1 for Gram positive), however, a similar concentration of F-AgNPs could effectively inhibit the growth (Table S1† and Table 1). The study suggests that the mixing of nanoparticles was unable to show a strong antibacterial activity, due to the presence of different surface areas and shapes. However, the individual nanoparticles, with large surface areas, have more potential towards inhibiting the growth of bacterial strains.
Fig. 5, represents the size dependent killing kinetics of S-AgNPs, O-AgNPs, R-AgNPs and F-AgNPs against the E. coli (A), P. aeruginosa (B), B. subtilis (C) and S. aureus (D) bacterial strains used in the earlier studies. In the case of Gram negative bacteria, F-AgNPs and R-AgNPs showed bacteriostatic (99.36% and 99.00% killing) and bactericidal effects (99.90% and 99.00% killing) in 60 and 90 minutes respectively, while, O-AgNPs and S-AgNPs displayed almost similar bactericidal efficacy and requires 120 minutes to achieve ∼99.9% bacterial reduction. This clearly indicates that the flower shaped AgNPs mediate the fastest bactericidal action by first quickly inhibiting bacterial proliferation within 60 minutes and thereafter achieving bacterial reduction over the next half an hour.
Similarly, for the Gram positive bacteria, F-AgNPs and R-AgNPs show antibacterial activity by achieving bacteriostatic (99.42% and 99.21% killing) and bactericidal effects (99.93% and 99.30% killing) within 90 and 120 minutes, respectively. Although, the similar efficacy for all bacterial strains was achieved in 120 minutes using O-AgNPs and S-AgNPs, out of the four bacterial strains tested, S. aureus was found to be the least sensitive against AgNPs. This can be explained on the basis of the time taken to achieve the bacteriostatic effect, which was extended from 60 minutes for F-AgNPs and R-AgNPs to 90 and 120 minutes for O-AgNPs and S-AgNPs, respectively.
We have also employed a bacterial colony count method to explore the real world applicability of the synthesized F-AgNPs. The water samples used in the experiment were collected from the local pond and river (Dhanbad, Jharkhand). The F-AgNPs (different concentrations) was incubated with 5.0 mL of the collected water sample for 15 min, then the nanoparticles were filtered from the solution and the remaining water sample was cultured on a LB plate for 24 h at 37 °C. The number of colonies grown on the surface of LB plate was used to monitor the bactericidal efficiency of the F-AgNPs. As expected, we found that the F-AgNPs have a very high bacterial capture ability and the bacterial cells were removed with 90–95% efficiency.
Conclusions
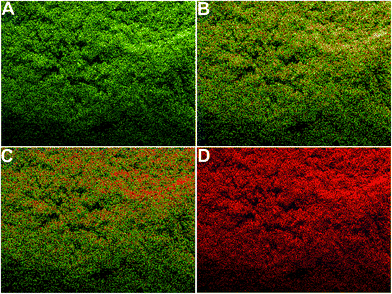
In summary, we represent an environmental friendly method to synthesize different shaped AgNPs with the help of pomegranate juice as a reducing agent and a microwave assisted method. No toxic reagents were used to synthesize the different shaped AgNPs (spherical, oval, rod and flower). All the AgNPs were found to be highly effective as antibacterial agents to the all studied bacterial strains and their antibacterial efficacy increased when the shapes of the AgNPs were changed (F-AgNPs > R-AgNPs > O-AgNPs > S-AgNPs). The bacteria killing efficiency was also visualized, herein, using confocal microscopic analysis. Additionally, the synthesized F-AgNPs were also applied to the capture and rapid removal of pathogenic bacteria from real water samples.Acknowledgements
The authors are thankful to the Department of Science and Technology, Government of India for the sanction of the Fast Track Research Project for Young Scientists to Dr Rashmi Madhuri (Ref. No.: SB/FT/CS-155/2012) and Dr Prashant K. Sharma (Ref. No.: SR/FTP/PS-157/2011). Dr Sharma (FRS/34/2012-2013/APH) and Dr Madhuri (FRS/43/2013-2014/AC) are also thankful to the Indian School of Mines, Dhanbad for a Major Research Project grant under the Faculty Research Scheme. We are also thankful to the Board of Research in Nuclear Sciences (BRNS), Department of Atomic Energy, Government of India, for the major research project (34/14/21/2014-BRNS).References
- J. J. Koh, S. Lin, T. T. Aung, F. Lim, H. Zou, Y. Bai, J. Li, H. Lin, L. M. Pang, W. L. Koh, S. M. Salleh, R. Lakshminarayanan, L. Zhou, S. Qiu, K. Pervushin, C. Verma, D. T. Tan, D. Cao, S. Liu and R. W. Beuerman, Amino acid modified xanthone derivatives: novel, highly promising membrane-active antimicrobials for multidrug-resistant Gram-positive bacterial infections, J. Med. Chem., 2015, 58, 739–752 CrossRef CAS PubMed.
- B. Nagy, A. Szmolka, S. Smole Možina, J. Kovač, A. Strauss, S. Schlager, J. Beutlich, B. Appel, M. Lušicky, P. Aprikian, J. Pászti, I. Tóth, R. Kugler and M. Wagner, Virulence and antimicrobial resistance determinants of verotoxigenic Escherichia coli (VTEC) and of multidrug-resistant E. coli from foods of animal origin illegally imported to the EU by flight passengers, Int. J. Food Microbiol., 2015, 209, 52–59 CrossRef CAS PubMed.
- D. Schoder, A. Strauß, K. Szakmary-Brändle, B. Stessl, S. Schlager and M. Wagner, Prevalence of major foodborne pathogens in food confiscated from air passenger luggage, Int. J. Food Microbiol., 2015, 209, 3–12 CrossRef PubMed.
- M. K. Rai, S. D. Deshmukh, A. P. Ingle and A. K. Gade, Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria, J. Appl. Microbiol., 2012, 112, 841–852 CrossRef CAS PubMed.
- H. Humberto, V. Lara, N. V. Ayala-Nunez, L. D. Carmen, T. Ixtepan and R. P. Cristina, Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria, World J. Microbiol. Biotechnol., 2010, 26, 615–621 CrossRef.
- L. Good and J. E. M. Stach, Synthetic RNA silencing in bacteria antimicrobial discovery and resistance breaking, Front. Microbiol., 2011, 2, 1–11 Search PubMed.
- S. Agnihotri, S. Mukherji and S. Mukherji, Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy, RSC Adv., 2014, 4, 3974–3983 RSC.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramirez and M. J. Yacaman, The bactericidal effect of silver nanoparticles, Nanotechnology, 2005, 16, 2346–2353 CrossRef CAS PubMed.
- C. Panneerselvam, S. Ponaruselvam and K. Murugan, Potential anti plasmodial activity of synthesized silver nanoparticles using Andrographis paniculata Nees (Acanthaceae), Arch. Appl. Sci. Res., 2011, 3, 208–217 CAS.
- K. RaoKudle, M. R. Donda, J. Alwala, R. Koyyati, V. Nagati, R. Merugu, Y. Prashanthi and M. P. P. Rudra, Biofabrication of silver nanoparticles using Cuminum cyminum through microwave irradiation, International Journal of Nanomaterials and Biostructures, 2012, 2, 65–69 Search PubMed.
- H. R. Ghorbani, A. A. Safekordi, H. Attar and S. M. Rezayat Sorkhabadi, Biological and non-biological methods for silver nanoparticles synthesis, Chem. Biochem. Eng. Q., 2011, 25, 317–326 CAS.
- S. Yeo, H. Lee and S. Jeong, Antibacterial effect of nanosized silver colloidal solution on textile fabrics, J. Mater. Sci., 2003, 38, 2143–2147 CrossRef CAS.
- J. Zhang, P. Chen, C. Sun and X. Hu, Sonochemical synthesis of colloidal silver catalysts for reduction of complexing silver in DTR system, Appl. Catal., A, 2004, 266, 49–54 CrossRef CAS.
- W. Zhang, X. Qiao, J. Chen and H. Wang, Preparation of silver nanoparticles in water-in-oil AOT reverse micelles, J. Colloid Interface Sci., 2006, 302, 370–373 CrossRef CAS PubMed.
- B. He, J. Tan, K. Liew and H. Liu, Synthesis of size controlled Ag nanoparticles, J. Mol. Catal. A: Chem., 2004, 221, 121–126 CrossRef CAS.
- M. Kühn, N. P. Ivleva, S. Klitzke, R. Niessner and T. Baumann, Investigation of coatings of natural organic matter on silver nanoparticles under environmentally relevant conditions by surface-enhanced Raman scattering, Sci. Total Environ., 2015, 535, 122–130 CrossRef PubMed.
- V. T. P. Vinod, P. Saravanan, B. Sreedhar, D. K. Devi and R. B. Sashidhar, A facile synthesis and characterization of Ag, Au and Pt nanoparticles using a natural hydrocolloid gum kondagogu (Cochlospermum gossypium), Colloids Surf., B, 2011, 83, 291–298 CrossRef CAS PubMed.
- P. Logeswari, S. Silambarasan and J. Abraham, Ecofriendly synthesis of silver nanoparticles from commercially available plant powders and their antibacterial properties, Sci. Iran., Trans. F, 2013, 20, 1049–1054 CAS.
- P. Banerjee, M. Satapathy, A. Mukhopahayay and P. Das, Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis, Biores. Bioproc., 2014, 1(3), 2–10 Search PubMed.
- S. Pal, Y. K. Tak and J. M. Song, Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli, Appl. Environ. Microbiol., 2007, 73, 1712–1720 CrossRef CAS PubMed.
- P. V. Dong, C. H. Ha, L. T. Binh and J. Kasbohm, Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles, Int. Nano Lett., 2012, 2, 1–9 CrossRef.
- J. Pal and M. K. Deb, Microwave synthesis of polymer coated silver nanoparticles by glucose as reducing agent, Indian J. Chem., 2012, 51, 821–824 Search PubMed.
- O. Fawole, N. P. Makunga and U. L. Opara, Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract, BMC Complementary Altern. Med., 2012, 12, 1–11 CrossRef PubMed.
- P. Yan, R. Wang, N. Zhao, H. Zhao, D.-F. Chene and F.-J. Xu, Polycation-functionalized gold nanoparticles with different morphologies for superior gene transfection, Nanoscale, 2015, 7, 5281–5291 RSC.
- S. Zhan, D. Zhu, S. Ma, W. Yu, Y. Jia, Y. Li, H. Yu and Z. Shen, Highly efficient removal of pathogenic bacteria with magnetic graphene composites, ACS Appl. Mater. Interfaces, 2015, 7, 4290–4298 CAS.
- P. Mulvaney, Surface plasmon spectroscopy of nanosized metal particles, Langmuir, 1996, 12, 788–800 CrossRef CAS.
- U. Kreibig and M. Vollmer, Optical properties of metal clusters, Springer, Berlin, Germany, 1995 Search PubMed.
- G. Mie, Contributions to the optics of turbid media, especially colloidal metal solutions, Ann. Phys., 1908, 25, 377–445 CrossRef CAS.
- P. Velusamy, J. Das, R. Pachaiappan, B. Vaseeharan and K. Pandian, Greener approach for synthesis of antibacterial silver nanoparticle using aqueous solution of neem gum (Azadirachta indica L.), Ind. Crops Prod., 2015, 66, 103–109 CrossRef CAS.
- J. Xu, G. Cheng and R. Zheng, Controllable synthesis of highly ordered Ag nanorod arrays by chemical deposition method, Appl. Surf. Sci., 2010, 256, 5006–5010 CrossRef CAS.
- Z. Zaheer and Rafiuddin, Multi-branched flower-like silver nanoparticles: preparation and characterization, Colloids Surf., A, 2011, 384, 427–431 CrossRef CAS.
- M. S. Bakshi, Room temperature surfactant assisted crystal growth of silver nanoparticles to nanoribbons, J. Nanosci. Nanotechnol., 2010, 10, 1757–1765 CrossRef CAS PubMed.
- X. W. Lou, C. Yuan and L. A. Archer, An unusual example of hyperbranched metal nanocrystals and their shape evolution, Chem. Mater., 2006, 18, 3921–3923 CrossRef CAS.
- R. Jin, Y. Cao, C. A. Mirkin, K. L. Kelly, G. C. Schatz and J. G. Zheng, Photoinduced conversion of silver nanospheres to nanoprisms, Science, 2001, 291, 1901–1903 CrossRef PubMed.
- M. R. El-Zahry, A. Mahmoud, I. H. Refaat, H. A. Mohamed, H. Bohlmann and B. Lendl, Antibacterial effect of various shapes of silver nanoparticles monitored by SERS, Talanta, 2015, 138, 183–189 CrossRef CAS PubMed.
- S. Zhan, Y. Yang, Z. Shen, J. Shan, Y. Li, S. Yang and D. Zhua, Efficient removal of pathogenic bacteria and viruses by multifunctional amine-modified magnetic nanoparticles, J. Hazard. Mater., 2014, 274, 115–123 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18575k |
| This journal is © The Royal Society of Chemistry 2015 |