Combining the PeT and ICT mechanisms into one chemosensor for the highly sensitive and selective detection of zinc†
Ting Wei,
Jinglu Wang,
Yu Chen and
Yifeng Han*
Department of Chemistry, The Key Laboratory of Advanced Textile Materials and Manufacturing Technology, Zhejiang Sci-Tech University, Hangzhou, 310018, China. E-mail: zstuchem@gmail.com; Tel: +86-751-86843550
First published on 16th June 2015
Abstract
A novel fluorescent sensor (ZS1) based on the dual-mechanism of PeT/ICT for the highly sensitive and selective detection of Zn2+ was designed and synthesized. ZS1 displays remarkable selectivity for Zn2+ with an enhanced red-shift in both absorption and emission, which results from the Zn2+-triggered deprotonation of its amide group. ZS1 could detect as low as 7.2 × 10−9 M Zn2+ with an association constant value of 6.27 × 104 M−1. More importantly, it displays specific and sensitive recognition of Zn2+ and especially avoids the interference of Cd2+ in aqueous solution. The probe was also demonstrated to detect Zn2+ in living cells.
Introduction
Zinc, which is widely distributed in air, water, and solids, is the second most abundant transition metal ion in organisms.1 Zinc plays crucial roles in many important biological processes such as structural and catalytic cofactors, neural signal transmitters, and gene expression regulators. The normal concentration range for zinc ions in biological systems is narrow, and both a deficiency and excess cause many pathological states such as Alzheimer's disease (AD), amyotrophic lateral sclerosis (ALS), epilepsy, Parkinson's disease, ischemic stroke, and infantile diarrhea.2 Accordingly, it is desirable to develop new analytical methods for the detection and monitoring of zinc ions in vitro and in vivo.In fact, numerous fluorescent sensors have been developed to detect and analyze zinc ions due to their simplicity, high sensitivity, and real-time detection.3 Unfortunately, only a few zinc ion fluorescent sensors that can distinguish Zn2+ from Cd2+ with high selectivity have been reported.4 Zn2+ and Cd2+ are in the same group of the periodic table and have similar properties, which cause similar spectral changes when they are coordinated with fluorescent sensors. Therefore, for practical applications, it is still strongly desirable to develop fluorescent chemosensors with excellent performance for Zn2+ under physiological conditions.
It should be noted that compared with a conventional one mechanism method such as photo-induced electron transfer (PeT),5 excited-state intramolecular proton transfer (ESIPT),6 intramolecular charge transfer (ICT),7 and aggregation-induced emission (AIE)8 based sensors, which usually give one single signaling response, the combination of two or more mechanisms based sensors would be more attractive since such a multi-mechanism will usually produce multiple signals to amplify recognition events to a greater extent and finally improve selectivity and sensitivity. To date, several fluorescent sensors have been developed on the basis of multi-mechanism. For example, Akkaya et al. reported BODIPY-derived probes for the sensing of Hg2+, GSH, and biological thiols based on the PeT/ICT dual-mechanism.9 Wang and Zhang et al. applied the PeT/ESIPT dual-mechanism to design a 3-hydroxyflavone based probe for thiols.10 However, the combination of PeT/ICT dual-mechanism based zinc sensors have been scarcely explored. Recently, Yoon, Shin, and Xu et al. developed a novel PeT/ICT dual-mechanism based fluorescent sensor by Zn2+-triggered amide tautomerization for the high selectivity of zinc,4a which is difficult to accomplish via a single mechanism.
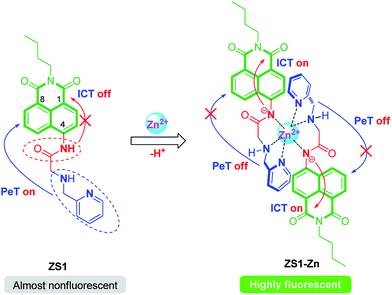
Inspired by all of these works, we report here a novel PeT/ICT dual-mechanism based fluorescence turn-on zinc sensor, ZS1, which is a 2-picolylamine (PA) derivative of 4-aminonaphthalimide, for the detection of Zn2+. In ZS1, an amide group has been inserted to link 1,8-naphthalimide fluorophore and PA chelator. We envision that the fluorescence of ZS1 would be quenched not only by the PeT effect from the PA chelator but also by the ICT effect from the 4-amide group. However, the capture of Zn2+ by the amide-PA receptor resulted in red shifts in both the absorption and fluorescence spectra due to the repressed PeT process from the N atoms of PA to the fluorophore and the enhanced ICT process from the N atom in the 4 position of ZS1 to 1,8-naphthalimide by the deprotonation of NH in the 4-amide group. This type of binary effect of the PeT/ICT mechanisms of Zn2+ to ZS1 exhibits high binding affinity and selectivity towards Zn2+ (Scheme 1).
Results and discussion
ZS1 can be readily prepared in two convenient steps under facile reaction conditions with a high yield starting with 4-amino-N-butyl-1,8-naphthalimide (1). The product (ZS1) was well characterized by 1H, 13C NMR, and HR-MS (Scheme 2). | ||
| Scheme 2 Synthesis of ZS1: (a) 2-chloroacetyl chloride/NEt3, DCM, reflux, 1 h, 89%; (b) 2-picolylamine/DIPEA/KI, CH3CN, reflux, 10 h, 48%. | ||
In its UV-vis absorption spectrum (Fig. 1), ZS1 exhibits a broad band from 300 to 450 nm with its maximum centered at 360 nm, which is assigned to the π–π* transitions of the 1,8-naphthalimide. Upon the addition of Zn2+ (0–10.0 equiv.), this band red-shifts to 366 nm, and is accompanied by three clear isosbestic points at 266, 342, and 407 nm, respectively, which are due to the deprotonation of the amide NH group, and this deprotonation process strengthens the electron-donating ability from the nitrogen atom of the 4-amide group to 1,8-naphthalimide. Furthermore, a good linear relationship (R2 = 0.994) was observed between the changes in the absorbance at 366 nm with Zn2+ in the range of 0–120.0 μM (Fig. S1, ESI†).
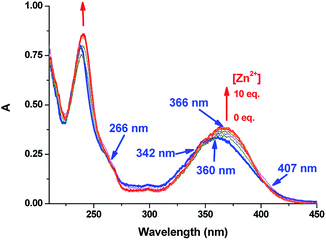 | ||
| Fig. 1 Absorption spectra of ZS1 (20.0 μM) in Tris-HCl buffer (10 mM, pH 7.2, containing 1% CH3CN) in the presence of different concentrations of Zn2+ (0–10.0 equiv.). | ||
As expected, ZS1 alone is weak blue fluorescent (ε = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 598 M−1 cm−1, Φ0 = 0.033, λex = 360 nm, λem = 465 nm, Table S1, ESI†) in neutral aqueous solution (10 mM Tris-HCl buffer, pH 7.2, containing 1% CH3CN). While the addition of 10.0 equiv. of Zn2+ induced a red-shift in the emission of ZS1 to 522 nm and triggered a ca. 5.3-fold (green fluorescence, ε = 12
598 M−1 cm−1, Φ0 = 0.033, λex = 360 nm, λem = 465 nm, Table S1, ESI†) in neutral aqueous solution (10 mM Tris-HCl buffer, pH 7.2, containing 1% CH3CN). While the addition of 10.0 equiv. of Zn2+ induced a red-shift in the emission of ZS1 to 522 nm and triggered a ca. 5.3-fold (green fluorescence, ε = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 145 M−1 cm−1, Φ0 = 0.175, λex = 360 nm, λem = 522 nm, Table S1, ESI†) increase in the integrated emission for ZS1 (Fig. 2a). These fluorescence behaviors were attributed to the coordination of the PA chelator and the deprotonated amide nitrogen of ZS1 with Zn2+, which simultaneously repressed the PeT effect from the PA chelator and enhanced the ICT process from the deprotonated 4-amide group. The titration of ZS1 with Zn2+ was followed by fluorescence to determine the ZS1/Zn2+ binding ratio and association constant (Ka). The Ka of ZS1/Zn2+ was determined to be 6.27 × 104 M−1 by the Hill plot analysis (Fig. 3a). Moreover, the Job's plot, which exhibits a maximum at 0.34 M fraction of Zn2+, indicated that a 2
145 M−1 cm−1, Φ0 = 0.175, λex = 360 nm, λem = 522 nm, Table S1, ESI†) increase in the integrated emission for ZS1 (Fig. 2a). These fluorescence behaviors were attributed to the coordination of the PA chelator and the deprotonated amide nitrogen of ZS1 with Zn2+, which simultaneously repressed the PeT effect from the PA chelator and enhanced the ICT process from the deprotonated 4-amide group. The titration of ZS1 with Zn2+ was followed by fluorescence to determine the ZS1/Zn2+ binding ratio and association constant (Ka). The Ka of ZS1/Zn2+ was determined to be 6.27 × 104 M−1 by the Hill plot analysis (Fig. 3a). Moreover, the Job's plot, which exhibits a maximum at 0.34 M fraction of Zn2+, indicated that a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex is formed between ZS1 and Zn2+ (Fig. 3b). We also carried out HPLC-MS measurements for the ZS1-Zn2+ solution (Fig. S3, ESI†). All the results agree well with the proposed structure of the ZS1–Zn2+ complex (Fig. S4, ESI†).
1 complex is formed between ZS1 and Zn2+ (Fig. 3b). We also carried out HPLC-MS measurements for the ZS1-Zn2+ solution (Fig. S3, ESI†). All the results agree well with the proposed structure of the ZS1–Zn2+ complex (Fig. S4, ESI†).
To clarify the actual ZS1/Zn2+ interaction, an 1H NMR titration experiment was conducted. As shown in Fig. 4, the two methylene protons at 3.97 and 3.56 ppm, which are attributed to the H-7′ and 8′, respectively, together with the pyridine protons (H-3′, 4′, 5′, 6′) were dramatically shifted downfield after the addition of Zn2+ due to the chelation of the lone-pair electrons of the two nitrogen atoms of PA by Zn2+. Moreover, the chemical shifts of five aromatic resonances, which are attributed to the 1,8-naphthalimide protons, experienced an opposite shift, thus indicating that the deprotonation of 4-amide group of ZS1 occurred in the presence of Zn2+. These results agree well with the optical responses.
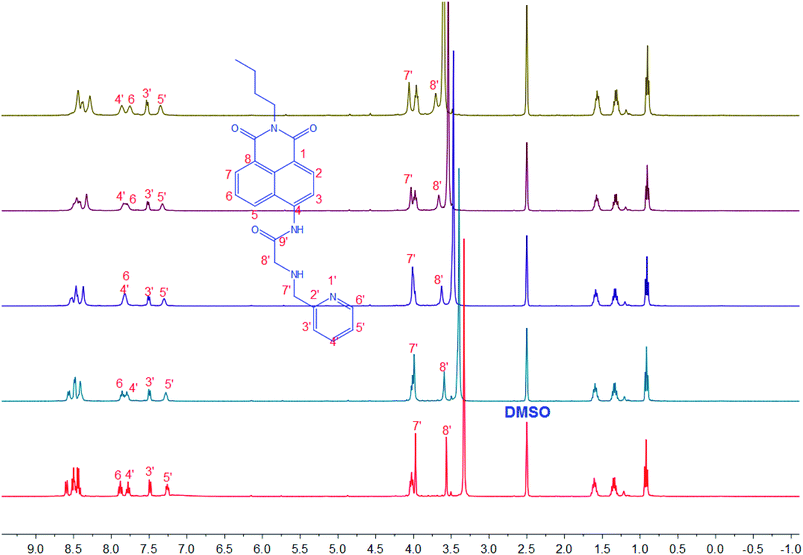 | ||
| Fig. 4 1H NMR titration experiment of ZS1 in the presence of different concentrations of Zn2+ (0–10.0 equiv. of Zn2+, in d6-DMSO). | ||
Furthermore, the fluorescence titration of ZS1 with various metal ions was conducted to examine its selectivity (Fig. 2b). Much to our delight, the examined alkali, alkaline-earth metal ions, and transition metal ions showed nominal changes in the fluorescence of spectra of ZS1. Competing experiments were then carried out in the presence of Zn2+ mixed with other competing metal ions (Fig. 5a and b). Except for Cu2+, other background metal ions had no obvious interference with the detection of Zn2+ ions. It should be noted that ZS1 displayed considerable ability to distinguish Zn2+ from Cd2+, which have properties similar to Zn2+ and generally cause strong interference.
pH effects on the fluorescence of ZS1 and the ZS1–Zn2+ system were also investigated. As depicted in Fig. 6, ZS1 alone is inert to pH in the range of 12.0–6.0, but the fluorescent intensity dramatically increased from pH 6.0 to 3.0 due to the inhibited PeT process by the protonation of the two nitrogen atoms of the PA chelator. However, satisfactory Zn2+-sensing abilities were exhibited in a range of pH from 6.0 to 9.0, which indicate that ZS1 could be used in neutral natural systems or in a mildly acidic or basic environment.
 | ||
| Fig. 6 Effect of the pH on the fluorescence emission of ZS1 (10.0 μM) alone and ZS1–Zn2+ system (10.0 μM of ZS1 in the presence of 10.0 equiv. of Zn2+), slit = 1.5/3. | ||
For practical purposes, the detection limit of ZS1 for the analysis of Zn2+ is also an important parameter. The fluorescence titration curve revealed that the fluorescence intensity of ZS1 at 522 nm increased linearly with the amount of Zn2+ in the range of 0–5.0 μM (R2 = 0.99) (Fig. S2, ESI†). Thus, the detection limit of ZS1 for Zn2+ was calculated to be 7.2 × 10−9 M, which reveals the high sensitivity for the analysis of zinc ions using ZS1.
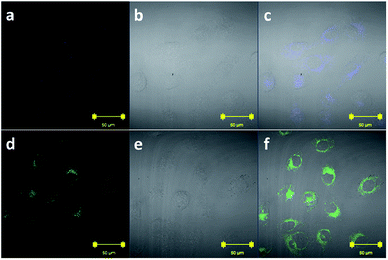
Due to the favorable properties of ZS1 in vitro, the potential utility of ZS1 in living cells was studied. HeLa cells that were incubated with 5.0 μM of ZS1 for 0.5 h at 37 °C exhibited weak blue fluorescence (Fig. 7a). The cells were then treated with ZnCl2 (5.0 μM) for 0.5 h at 37 °C and this resulted in a dramatic increase of intracellular green fluorescence (Fig. 7d), which indicated that ZS1 was cell membrane permeable and capable of imaging Zn2+ in living cells.
Conclusion
In conclusion, we successfully developed a novel PeT/ICT dual-mechanism based fluorescent probe, ZS1, for the selective detection of Zn2+ in an aqueous solution. ZS1 displays excellent fluorescent selectivity for Zn2+ with an enhanced red-shift in both absorption and emission, which results from the Zn2+-triggered deprotonation of its amide group. Moreover, based-on this PeT/ICT dual-mechanism, ZS1 can easily distinguish Zn2+ from Cd2+ in an aqueous solution, which is usually a technical problem for other related probes. Furthermore, the fluorescence imaging of Zn2+ in living cells indicated that this probe might be favorable for biological applications. We anticipate that the experimental results of this study will inspire the future designing of metal-ion sensors in water for a variety of chemical and biological applications.Experimental section
Materials and measurements
All solvents were of analytical grade. NMR experiments were carried out on a Bruker AV-400 NMR spectrometer with chemical shifts reported in ppm (in CDCl3, d6-DMSO or TMS as an internal standard). The mass spectrum (MS) was obtained on a SHIMADZU LCMS-2020 spectrometer. All pH measurements were made with a Sartorius basic pH-Meter PB-10. Fluorescence spectra were obtained on a PerkinElmer LS55 Fluorescence spectrophotometer. Absorption spectra were obtained on a Shimadzu UV 2501(PC)S UV-Visible spectrophotometer. Unless otherwise noted, the excitation and emission widths for ZS1 were all 3.Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) as the eluant to give 2 as a pale yellow solid (580 mg, 89%); 1H NMR (400 MHz, CDCl3) δ 9.14 (brs, 1H), 8.64 (m, 2H), 8.48 (d, J = 7.9 Hz, 1H), 8.19 (d, J = 8.4 Hz, 1H), 7.83 (t, J = 7.7 Hz, 1H), 4.40 (s, 2H), 4.18 (t, J = 7.2 Hz, 2H), 1.71 (m, 2H), 1.45 (m, 2H), 0.98 (t, J = 7.1 Hz, 3H).
2, v/v) as the eluant to give 2 as a pale yellow solid (580 mg, 89%); 1H NMR (400 MHz, CDCl3) δ 9.14 (brs, 1H), 8.64 (m, 2H), 8.48 (d, J = 7.9 Hz, 1H), 8.19 (d, J = 8.4 Hz, 1H), 7.83 (t, J = 7.7 Hz, 1H), 4.40 (s, 2H), 4.18 (t, J = 7.2 Hz, 2H), 1.71 (m, 2H), 1.45 (m, 2H), 0.98 (t, J = 7.1 Hz, 3H).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dichloromethane
1 dichloromethane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol); m.p. = 131–132 °C; 1H NMR (400 MHz, CDCl3) δ 10.99 (brs, 1H), 8.68 (d, J = 8.2 Hz, 1H), 8.61 (m, 2H), 8.55 (d, J = 4.7 Hz, 1H), 8.43 (d, J = 8.5 Hz, 1H), 7.75 (t, J = 7.9 Hz, 1H), 7.69 (td, J = 7.7, 1.6 Hz, 1H), 7.27–7.18 (m, 2H), 4.17 (t, J = 7.2 Hz, 2H), 4.10 (s, 2H), 3.64 (s, 2H), 1.72 (m, 2H), 1.45 (m, 2H), 0.98 (t, J = 7.3 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 170.40, 164.25, 163.69, 157.81, 149.73, 138.78, 136.85, 132.67, 131.02, 128.94, 126.53, 123.25, 122.72, 122.52, 118.02, 117.03, 54.99, 53.13, 40.20, 30.22, 20.38, 13.82; HR-MS (ESI-TOF): m/z 417.1960 [M + H]+, calc'd 417.1927.
methanol); m.p. = 131–132 °C; 1H NMR (400 MHz, CDCl3) δ 10.99 (brs, 1H), 8.68 (d, J = 8.2 Hz, 1H), 8.61 (m, 2H), 8.55 (d, J = 4.7 Hz, 1H), 8.43 (d, J = 8.5 Hz, 1H), 7.75 (t, J = 7.9 Hz, 1H), 7.69 (td, J = 7.7, 1.6 Hz, 1H), 7.27–7.18 (m, 2H), 4.17 (t, J = 7.2 Hz, 2H), 4.10 (s, 2H), 3.64 (s, 2H), 1.72 (m, 2H), 1.45 (m, 2H), 0.98 (t, J = 7.3 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 170.40, 164.25, 163.69, 157.81, 149.73, 138.78, 136.85, 132.67, 131.02, 128.94, 126.53, 123.25, 122.72, 122.52, 118.02, 117.03, 54.99, 53.13, 40.20, 30.22, 20.38, 13.82; HR-MS (ESI-TOF): m/z 417.1960 [M + H]+, calc'd 417.1927.Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 20902082), the Zhejiang Provincial Natural Science Foundation of China (Grant No. Y3100416) and the Program for Innovative Research Team of Zhejiang Sci-Tech University (13060052-Y).Notes and references
- (a) P. Jiang and Z. Guo, Coord. Chem. Rev., 2004, 248, 205 CrossRef CAS PubMed; (b) J. M. Berg and Y. Shi, Science, 1996, 271, 1081 CAS; (c) X. Xie and T. G. Smart, Nature, 1991, 349, 521 CrossRef CAS PubMed; (d) B. L. Vallee and K. H. Falchuk, Physiol. Rev., 1993, 73, 79 CAS; (e) K. H. Falchuk, Mol. Cell. Biochem., 1998, 188, 41 CrossRef CAS.
- (a) A. I. Bush, Curr. Opin. Chem. Biol., 2000, 4, 184 CrossRef CAS; (b) A. I. Bush, Trends Neurosci., 2003, 26, 207 CrossRef CAS; (c) M. P. Cuajungco and G. J. Lees, Brain Res. Rev., 1997, 23, 219 CrossRef CAS.
- (a) Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16 RSC; (b) Z. Liu, W. He and Z. Guo, Chem. Soc. Rev., 2013, 42, 1568 RSC; (c) L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622 RSC; (d) G. Zhang, H. Li, S. Bi, L. Song, Y. Lu, L. Zhang, J. Yu and L. Wang, Analyst, 2013, 138, 6163 RSC; (e) S. Jiao, L. Peng, K. Li, Y. Xie, M. Ao, X. Wang and X. Yu, Analyst, 2013, 138, 5762 RSC; (f) P. Li, X. Zhou, R. Huang, L. Yang, X. Tang, W. Dou, Q. Zhao and W. Liu, Dalton Trans., 2014, 706 RSC; (g) Y. Zhang, X. F. Guo, W. X. Si, L. H. Jia and X. H. Qian, Org. Lett., 2008, 10, 473 CrossRef CAS PubMed; (h) A. Ajayaghosh, P. Carol and S. Sreejith, J. Am. Chem. Soc., 2005, 127, 14962 CrossRef CAS PubMed; (i) D. A. Pearce, N. Jotterand, I. S. Carrico and B. Imperiali, J. Am. Chem. Soc., 2001, 123, 5160 CrossRef CAS; (j) S. C. Burdette, C. J. Frederickson, W. Bu and S. J. Lippard, J. Am. Chem. Soc., 2003, 125, 1778 CrossRef CAS PubMed; (k) Z. Xu, X. Liu, J. Pan and D. R. Spring, Chem. Commun., 2012, 48, 4764 RSC; (l) Y. Ding, Y. Xie, X. Li, J. P. Hill, W. Zhang and W. Zhu, Chem. Commun., 2011, 47, 5431 RSC.
- (a) Z. Xu, K.-H. Baek, H. N. Kim, J. Cui, X. Qian, D. R. Spring, I. Shin and J. Yoon, J. Am. Chem. Soc., 2010, 132, 601–610 CrossRef CAS PubMed; (b) Z. Xu, X. Qian, J. Cui and R. Zhang, Tetrahedron, 2006, 62, 10117–10122 CrossRef CAS PubMed; (c) L. Xue, C. Liu and H. Jiang, Org. Lett., 2009, 11, 1655–1658 CrossRef CAS PubMed; (d) Y. Tan, J. Gao, J. Yu, Z. wang, Y. Cui, Y. Yang and G. Qian, Dalton Trans., 2013, 11465–11470 RSC; (e) P. Li, X. Zhou, R. huang, L. Yang, X. Tang, W. Dou, Q. Zhao and W. Liu, Dalton Trans., 2014, 706–713 RSC; (f) K. Wu, Y. Gao, Z. Yu, F. Yu, J. Jiang, J. Guo and Y. Han, Anal. Methods, 2014, 6, 3560–3563 RSC; (g) M. Khan, C. R. Goldsmith, Z. Huang, J. Georgiou, T. T. Luyben, J. C. Roder, S. J. Lippard and K. Okamoto, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 6786–6791 CrossRef CAS PubMed; (h) T. Mistri, M. Dolai, D. Chakraborty, A. R. Khuda-Bukhsh, K. K. Das and M. Ali, Org. Biomol. Chem., 2012, 10, 2380–2384 RSC; (i) G. Sivaraman, T. Anand and D. Chellappa, Analyst, 2012, 137, 5881–5884 RSC; (j) J. F. Zhu, W. H. Chan and A. W. M. Lee, Tetrahedron Lett., 2012, 53, 2001–2004 CrossRef CAS PubMed; (k) L. Praveen, C. H. Suresh, M. L. P. Reddy and R. L. Varma, Tetrahedron Lett., 2011, 52, 4730–4733 CrossRef CAS PubMed; (l) B. K. Datta, S. Mukherjee, C. Kar, A. Ramesh and G. Das, Anal. Chem., 2013, 85, 8369–8375 CrossRef CAS PubMed.
- (a) T. Matsumoto, Y. Urano, T. Shoda, H. Kojima and T. Nagano, Org. Lett., 2007, 9, 3375 CrossRef CAS PubMed; (b) L.-Y. Lin, X.-Y. Lin, F. Lin and K.-T. Wong, Org. Lett., 2011, 13, 2216 CrossRef CAS PubMed; (c) Y. Gabe, Y. Urano, K. Kikuchi, H. Kojima and T. Nagano, J. Am. Chem. Soc., 2004, 126, 3357 CrossRef CAS PubMed; (d) J. Wang and X. Qian, Chem. Commun., 2006, 109 RSC; (e) J. Wang, X. Qian and J. Cui, J. Org. Chem., 2006, 71, 4308 CrossRef CAS PubMed; (f) J. Wang and X. Qian, Org. Lett., 2006, 8, 3721 CrossRef CAS PubMed; (g) J. Liu, K. Wu, S. Li, T. Song, Y. Han and X. Li, Dalton Trans., 2013, 3854 RSC.
- (a) T. I. Kim, H. J. Kang, G. Han, S. J. Chung and Y. Kim, Chem. Commun., 2009, 5895 RSC; (b) R. Hu, J. A. Feng, D. H. Hu, S. Q. Wang, S. Y. Li, Y. Li and G. Q. Yang, Angew. Chem., Int. Ed., 2010, 49, 4915 CrossRef CAS PubMed; (c) M. Santra, B. Roy and K. H. Ahn, Org. Lett., 2011, 13, 3422 CrossRef CAS PubMed; (d) Z. Xu, L. Xu, J. Zhou, Y. Xu, W. Zhu and X. Qian, Chem. Commun., 2012, 48, 10871 RSC; (e) S. Chen, P. Hou, J. Wang and X. Song, RSC Adv., 2012, 2, 10869 RSC; (f) M. Kumar, N. Kumar and V. Bhalla, RSC Adv., 2013, 3, 1097 RSC.
- (a) D. Srikun, E. W. Miller, D. W. Domaille and C. J. Chang, J. Am. Chem. Soc., 2008, 130, 4596 CrossRef CAS PubMed; (b) L. Yuan, W. Lin, J. Song and Y. Yang, Chem. Commun., 2011, 47, 12691 RSC; (c) F. Yu, P. Li, P. Song, B. Wang, J. Zhao and K. Han, Chem. Commun., 2012, 48, 2852 RSC; (d) X. Li, S. Zhang, J. Cao, N. Xie, T. Liu, B. Yang, Q. He and Y. Hu, Chem. Commun., 2013, 49, 8656 RSC; (e) J. Liu, K. Wu, X. Li, Y. Han and M. Xia, RSC Adv., 2013, 3, 8924 RSC.
- (a) L. Peng, Z. Zhou, R. Wei, K. Li, P. Song and A. Tong, Dyes Pigm., 2014, 108, 24 CrossRef CAS PubMed; (b) X. Li, C. Yang, K. Wu, Y. Hu, Y. Han and S. H. Liang, Theranostics, 2014, 4, 1233 CrossRef CAS PubMed; (c) H. Wang, Y. Huang, X. Zhao, W. Gong, Y. Wang and Y. Cheng, Chem. Commun., 2014, 50, 15075 RSC; (d) G. N. Zhao, B. Tang, Y. Q. Dong, W. H. Xie and B. Z. Tang, J. Mater. Chem. B, 2014, 2, 5093 RSC; (e) R. T. K. Kwok, C. W. T. Leung, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2015, 44, 4228 RSC.
- (a) O. A. Bozdemir, R. Guliyev, O. Buyukcakir, S. Selcuk, S. Kolemen, G. Gulseren, T. Nalbantoglu, H. Boyaci and E. U. Akkaya, J. Am. Chem. Soc., 2010, 132, 8029 CrossRef CAS PubMed; (b) M. Isik, R. Guliyev, S. Kolemen, Y. Altay, B. Senturk, T. Tekinay and E. U. Akkaya, Org. Lett., 2014, 16, 3260 CrossRef CAS PubMed; (c) M. Isik, T. Ozdemir, I. S. Turan, S. Kolemen and E. U. Akkaya, Org. Lett., 2013, 15, 216 CrossRef CAS PubMed.
- M. Lan, J. Wu, W. Liu, H. Zhang, W. Zhang, X. Zhuang and P. Wang, Sens. Actuators, B, 2011, 156, 332 CrossRef CAS PubMed.
- B. Liu and H. Tian, Chem. Commun., 2005, 3156 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, synthetic details of MS1, additional spectroscopic data, and copies of NMR spectra. See DOI: 10.1039/c5ra11194c |
| This journal is © The Royal Society of Chemistry 2015 |