Molecular dynamics simulation of the diffusion behavior of water in poly(vinylidene fluoride)/silica hybrid membranes†
Ruibing Bai,
Huixia Wang,
Pan Zhang,
Bo Xiao,
Bo Jiang and
Ge Zhou*
Key Laboratory of Green Chemistry and Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu 610065, P. R. China. E-mail: zhougekk@scu.edu.cn
First published on 17th June 2015
Abstract
A molecular dynamics (MD) simulation is employed to investigate the effects of the concentration and size of silica particles on the diffusion behavior of water in poly(vinylidene fluoride) (PVDF)/SiO2 hybrid membranes. The membranes with different concentrations of SiO2 particles (class I) and with different sizes of SiO2 particles (class II) are designed and studied. The X-ray diffraction patterns and mean-square displacement (MSD) values of PVDF, the fractional free volume (FFV) characteristics and diffusion coefficients of water are discussed. The results revealed that the incorporation of silica particles into PVDF increased the polymer chain mobility and FFV in PVDF/SiO2 hybrid membranes. The diffusion coefficient of water presented an increase as the concentration of SiO2 increased (0–14.6 wt%). With the same concentration of SiO2, when the sizes of the SiO2 particles are different, the diffusion coefficient of water presented an increase first and then decreased as the size of the silica increased (radius: 0.794–1.26 nm). When the radius of the silica is nearly 1 nm, the diffusion coefficient of water in the membranes is at its largest (4.50 × 10−12 m2 s−1).
1 Introduction
Organic–inorganic hybrid membranes are fabricated by blending inorganic particles with organic polymer membranes. They have many interesting properties which are not found in pure polymer membranes,1–4 such as transport properties, as well as chemical and thermal stability. They can be widely used in the fields of automobiles, aeroplanes, electronics, architecture, new process and highly effective catalysts, environment-protecting materials, and so on. Their versatility is mainly due to the presence of inorganic filler materials, which can alter the mechanical properties of the polymer material significantly.5–7 Organic–inorganic hybrid membranes have attracted intense interest as potentially “the next generation” of membrane materials.8 Thus, it is necessary to understand the relations between the structural characteristics of hybrid membranes and their properties. Much effort has been devoted to experimental investigations. However, little has been reported about the theoretical analysis of the morphology upon the transport properties of small molecules in the hybrid membranes. One possible reason is that it is difficult to obtain accurate information using experimental technologies because of the restriction of the limited volume of the interfacial region.8It has been validated that molecular dynamics (MD) simulations are able to reveal the microstructure of hybrid membranes and provide the dynamics of the permeating compounds at the molecular level. It is possible that the diffusion behavior of small molecules is estimated in the interfacial region at the molecular level via MD simulation techniques. Some researchers have investigated the effects of inorganic particles on the interfacial morphology and the subsequent influence on diffusion properties. Tamai9–11 studied the diffusion process of methane, water, and ethanol in PDMS and polyethylene using MD simulations. Matthias Heuchel et al.12 analyzed the diffusion behaviors of oxygen, nitrogen, methane, and carbon dioxide through polyimide membranes using molecular simulations. Sakintuna et al.13 measured the diffusion coefficients and modes of transport of methanol, ethanol, n-propanol, 2-propanol and n-butanol through a zeolite. However, there is little literature devoted to the investigation of the diffusion behavior of water in PVDF/SiO2 hybrid membranes.
PVDF is one of the most extensively applied membrane materials in industry, because of its outstanding antioxidation properties, superior thermal and hydrolytic stabilities, as well as good mechanical and membrane forming properties. However, its hydrophobic nature that often results in severe membrane fouling and decline of permeability, has been a barrier for its application in water treatment.14 Inorganic materials that could be blended with PVDF include titanium dioxide (TiO2),15 zirconium dioxide (ZrO2),16 alumina (Al2O3)17 and some small molecule inorganic salts, such as lithium salts.18 Among the numerous inorganic materials, silica (SiO2) is the most convenient and widely used because of its mild reactivity and well-known chemical properties.19 Compared with the pure PVDF membrane, PVDF/SiO2 hybrid membranes have many outstanding properties, such as high-porosity and high intensity.
In this work, the effects of inorganic SiO2 particles on the diffusion properties of small penetrant molecules in PVDF/SiO2 hybrid membranes are investigated using MD simulations. It should be mentioned that silica particles have an α-quartz crystal structure, which is impermeable for the penetrant in order to exclude the influence of connected channels within the inorganic particles. The X-ray diffraction patterns, chain mobility, free volume characteristics and the diffusion coefficient of water in the PVDF control membrane and hybrid membranes were studied using MD simulations.
2 Simulation details
Molecular dynamics (MD) simulations were performed using the Materials Studio (version: 4.2) package, which was developed by Accelrys Software Inc. Energy minimization was conducted using the smart minimizer method which switched from steepest-descent to conjugated gradient and then to the Newton method as the energy derivatives decrease in order to accelerate the computation. The Andersen23 and Berendsen24 methods were employed to control temperature and pressure, respectively. A non-bond cutoff distance of 9.5 Å was employed to evaluate the non-bond interaction. The spline width and buffer width for the cutoff distance were 1.0 Å and 0.5 Å, respectively. The time step was set as 1.0 fs for all dynamics runs.The COMPASS20–22 (condensed phase optimization molecular potentials for atomistic simulation studies) module was applied in this work. The COMPASS force field is the first ab initio force field that has been parameterized and validated using condensed-phase properties in addition to various ab initio and empirical data for the molecules in isolation. The electrostatic potential energy was calculated using the Ewald25 summation technique and the van der Waals potential energy was calculated using the atom based26 technique.
The PVDF polymer chain consists of 100 repeat units. The silica particle with an approximate radius of 0.794 nm was constructed using the α-quartz silica crystal structure imported from the MS package, which was named as SiO2-1 (Fig. 1a). Two atactic PVDF chains and a number of silica particles were embedded into the cubic cells by using the Amorphous Cells Construction tools, which are filled with 5 water molecules with approximate 1.77 g cm−3 density to form all kinds of initial polymer membranes. An initial energy minimization was carried out at the initial stage to eliminate the bad contact. Cell samples were designated as PVDF, PVDF1SiO2-1, PVDF2SiO2-1, and PVDF4SiO2-1 (Fig. 1d–g) respectively according to the number of silica particles in the PVDF unit, marked as class I. In class I, PVDF, PVDF1SiO2-1, PVDF2SiO2-1, and PVDF4SiO2-1 correspond to the different concentrations of 0, 3.7, 7.3 and 14.6 wt%, respectively. In order to research the effect of the size of SiO2, another two silica particles, SiO2-2 and SiO2-4 (Fig. 1b and c), were constructed respectively. SiO2-2 is twice the volume of SiO2-1, and SiO2-4 is four times the volume of SiO2-1. The three particles possess a radius of 0.794, 1.00 and 1.26 nm respectively. Then another kind of model marked as class II is designed, which includes PVDF, PVDF4SiO2-1, PVDF2SiO2-2, and PVDF1SiO2-4 (Fig. 1d, g–i). In class II, except PVDF, the other three models possess the same concentration of 14.6 wt% and different size of SiO2 particles.
At the beginning of the simulation, the discover minimizer was rerun several times to get a more stabilized configuration, the energy vs. iteration curves are shown in Fig. 2. Then a 200 ps NPT28 (T = 298 K, P = 1.01 × 105 Pa) equilibration was adopted to obtain the justified density, followed by 1000 ps NVT27 (T = 298 K) dynamics. The temperature was controlled using the Andersen thermostat method and the pressure was controlled by the Berendsen method. The vdW interactions were calculated by using the atom based method with the cutoff distance set to 9.5 Å, and the coulomb interactions were calculated by using the Ewald method. The trajectories were saved every 1000 steps for further analysis.
 | ||
| Fig. 2 Energy vs. iteration (left: the first run of the discover minimizer; right: the last run of the discover minimizer). | ||
3 Results and discussion
3.1 Class I: different concentrations of SiO2
In class I, SiO2-1 was employed to construct models PVDF1SiO2-1, PVDF2SiO2-1, and PVDF4SiO2-1, which correspond to the different concentrations of 0, 3.7, 7.3, 14.6 wt%, respectively. In this part, the influence of different concentrations of silica on interfacial morphology and transport properties were studied.
 | (1) |
The simulated diffraction patterns of the hybrid membranes are shown in Fig. 3. It was shown that the PVDF control membrane and PVDF/SiO2 hybrid membranes generally existed in the amorphous state. It was also noted that the hybrid membranes exhibit a typical peak at about 34.92°, which is quite similar to the PVDF control membrane. It can be found that the peak intensity of the membranes follows the order of PVDF > PVDF1SiO2-1 > PVDF2SiO2-1 > PVDF4SiO2-1 from Fig. 3. This revealed that the intensity of the peak decreases while the mass fraction of SiO2 increases. It also can be found that the peak intensity of PVDF4SiO2-1 is apparently weaker than the peak intensity of the other three membranes (PVDF, PVDF1SiO2-1, PVDF2SiO2-1). The content of the hybrid membrane of PVDF4SiO2-1 is four times the nanoparticles of SiO2-1, and the concentration of SiO2-1 is 14.6 wt%. First, each of the four silica nanoparticles has a large interaction, which can deduce the apparent peak intensity of the PVDF4SiO2-1. Secondly, there is an interaction among the four silica nanoparticles, which may be a factor to decrease the peak intensity of PVDF4SiO2-1. Generally speaking, the PVDF/SiO2 hybrid membranes possessed less crystalline domains as the silica concentration increased, which is beneficial to the transport of small penetrants. The crystalline regions where the polymer chains are orderly packed are disrupted owing to interaction between the silica particles and polymer chains.
The mobility of the polymer is studied by examining the mean-square displacement (MSD) of the polymer chain.
| 〈Δr(t)2〉 = 〈(ri(t) − ri(0))2〉 | (2) |
The MSD of the polymer chain in the PVDF/SiO2 hybrid membranes is displayed in Fig. 4. The larger the slope of the MSD curve is, the stronger the chain mobility is. From Fig. 4, it is shown that the mobility of the polymer chain in hybrid membranes is stronger than that in the PVDF control membrane. In PVDF4SiO2-1 hybrid membranes, because of the higher concentration of silica particles and fewer crystalline domains, the polymer chain exhibits stronger mobility.
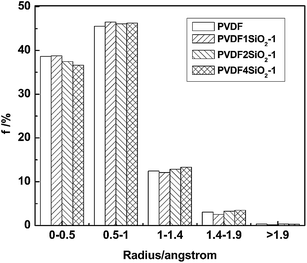
The free volume of the PVDF/SiO2 and pure PVDF membranes was characterized using a hard spherical probe. The Connolly surface (also called the molecular surface, shown in Fig. 5) is calculated when the probe molecule with the radius Rp rolls over the van der Waals surface, and the free volume is defined as the volume on the side of the Connolly surface without atoms. The FFV is determined by the ratio of the free volume to the total volume of the model. It should be mentioned that the free volume obtained by the probe method excludes the volume which is inaccessible to the probe. The free volume plays a crucial role in the transport behavior of penetrant molecules in membranes. The larger the fractional free volume, the better the free volume connectivity will be, and the faster the diffusion rate of small penetrants will be. The penetrant molecules are modeled by spheres with a radius of 0, 0.5, 1.0, 1.40 and 1.90 Å (collision radius). It should be mentioned that the free volume obtained using the probe method excludes the volume which is inaccessible to the probe.
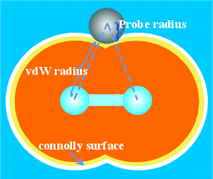 | ||
| Fig. 5 Definition of the Connolly surface. (M. L. Connolly, Science, 1983, 221, 709; M. L. Connolly, J. Appl. Crystal., 1983, 16, 548.) | ||
The results of the FFV, which depends on the radius of the probe, are shown in Table 1. The obtained results imply that the FFV drops sharply as the probe size increased. When the radius is 0 Å, the FFV of PVDF/SiO2 hybrid membranes are larger than that of the pure PVDF membrane and follow the order of PVDF4SiO2-1 > PVDF2SiO2-1 > PVDF1SiO2-1 > PVDF; when the radius is 0.5, 1.0 and 1.4 Å, the FFVs of the PVDF/SiO2 hybrid membranes are larger than that of the pure PVDF membrane and follow the order of PVDF4SiO2-1 > PVDF2SiO2-1 > PVDF1SiO2-1, but the FFVs of the PVDF/SiO2 hybrid membranes are not larger than that of the pure PVDF membrane; however, when the radius is 1.9 Å, the changes of the FFV of the PVDF/SiO2 hybrid membranes don’t show an obvious regulation. The specific reasons for this need be studied further in future work. It can be explained that when the silica particles are embedded in a glassy polymer, a gap appears at the organic–inorganic interface and its width depends greatly on the rigidity of the polymer and particles as well as the interaction between them.35 Thus, there are more free volume voids exhibited in PVDF4SiO2-1. A large amount of voids in the polymer bulk decreases the interchain interaction of the polymer and therefore enlarges the free volume voids in the polymer.
| Radius of the probe (Å) | 0 | 0.5 | 1.0 | 1.4 | 1.9 |
|---|---|---|---|---|---|
| PVDF | 24.09 | 14.79 | 3.83 | 0.82 | 0.08 |
| PVDF1SiO2-1 | 24.15 | 14.79 | 3.57 | 0.64 | 0.03 |
| PVDF2SiO2-1 | 24.26 | 15.18 | 4.00 | 0.88 | 0.09 |
| PVDF4SiO2-1 | 24.45 | 15.49 | 4.18 | 0.93 | 0.07 |
f is defined as the ratio of the FFVs as shown in eqn (3):
 | (3) |
 | (4) |
In this part, penetrant molecules (five water molecules) were inserted into the models. The diffusion coefficients were an averaged value from all penetrant molecules and were investigated under the NVT (T = 298 K) condition for 2000 ps. The diffusion coefficients of water are listed in Table 2. It was shown that an increase of the diffusion coefficients of water increased the mass fraction of silica particles. The chain mobility and the free volume are two key factors that affect the penetrant diffusivity in the membranes. A higher chain mobility induces a higher frequency of diffusion paths forming and disappearing. Higher FFVs always mean more accessible free volume voids in the membranes for the penetrant diffusion. Therefore, the diffusion coefficient of water in the PVDF4SiO2-1 membrane is higher than that of the other three membranes.
| Membranes | Diffusion coefficient |
|---|---|
| PVDF | 1.80 |
| PVDF1SiO2-1 | 2.77 |
| PVDF2SiO2-1 | 2.67 |
| PVDF4SiO2-1 | 3.73 |
3.2 Class II: different size of SiO2
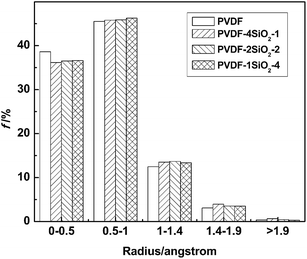
In class II, SiO2-1, SiO2-2, and SiO2-4 were employed to construct PVDF4SiO2-1, PVDF2SiO2-2, and PVDF1SiO2-4. The volume of SiO2-2 is twice that of SiO2-1 and SiO2-4 is four times that of SiO2-1. The concentration of silica is the same in these three hybrid membranes. In this part, the influences of the different sizes of silica on the interfacial morphology and transport properties of the membranes are studied.| Radius of the probe (Å) | 0 | 0.5 | 1.0 | 1.4 | 1.9 |
|---|---|---|---|---|---|
| PVDF | 24.09 | 14.79 | 3.83 | 0.82 | 0.08 |
| PVDF4SiO2-1 | 24.45 | 15.49 | 4.18 | 0.93 | 0.07 |
| PVDF2SiO2-2 | 24.56 | 15.59 | 4.33 | 0.97 | 0.10 |
| PVDF1SiO2-4 | 24.56 | 15.68 | 4.44 | 1.12 | 0.16 |
The ratios of different radii of FFVs are shown in Fig. 9. The fraction of f0–0.5 of the PVDF control membrane is larger than the hybrid membranes of class II. The fraction of FFV0.5–1 is increased as the size of the silica is increased. It is easy to find out that PVDF1SiO2-4 and PVDF2SiO2-2 have larger size free volume voids. This can explain what we studied in 3.2.1, that PVDF1SiO2-4 and PVDF2SiO2-2 hybrid membranes possess fewer crystalline domains than the other two membranes.
| Membranes | Diffusion coefficient |
|---|---|
| PVDF | 1.80 |
| PVDF4SiO2-1 | 3.73 |
| PVDF2SiO2-2 | 4.50 |
| PVDF1SiO2-4 | 2.23 |
4 Conclusion
Two series of models (class I and class II) were constructed to investigate the effects of silica particles on the diffusion properties of water molecules in PVDF/SiO2 membranes. The X-ray diffraction patterns, MSDs of polymer segments, FFVs of the hybrid membranes, and diffusion coefficients of water in the hybrid membranes were systematically investigated. It was observed that the diffusion coefficients of water in the membranes were larger than those in the control membrane. When the concentration of silica particles is different (class I), on the whole, the diffusion coefficient of water presented an increase as the concentration of silica increased (0–14.6 wt%). When the concentration range is from 3.7 to 7.3 wt%, the change of the diffusion coefficient is not obvious. With the same concentration of SiO2 particles, when the size of the silica particles is different (class II), the diffusion coefficient of water presented an increase first and then decreases as the increase of the size of silica (radius: 0.8–1.3 nm). When the diameter of the silica is nearly 1 nm, the diffusion coefficient of water in the membranes is the largest (4.50 × 10−12 m2 s−1). Furthermore, the results showed that the diffusion coefficients of water are positively correlated with the mobility of the PVDF chain and the free volume of the membranes; the diffusion coefficients of water are negatively correlated with the crystalline domains. Overall, MD simulations are a powerful tool to provide some theoretical details concerning the diffusion behavior of penetrant molecules in hybrid membranes, acting as a useful supplement and guidance to experimental investigation.References
- S. Y. Kwak, S. H. Kim and S. S. Kim, Environ. Sci. Technol., 2001, 35, 2388–2394 CrossRef CAS.
- R. K. Nagarale, G. S. Gohil, V. K. Shahi and R. Rangarajan, Macromolecules, 2004, 37, 10023–10030 CrossRef CAS.
- F. Peng, L. Lu, H. Sun, F. Pan and Z. Jiang, Ind. Eng. Chem. Res., 2007, 46, 2544–2549 CrossRef CAS.
- Y. Jia, Y. Zhang, R. Wang, J. Yi, X. Feng and Q. Xu, Ind. Eng. Chem. Res., 2012, 51, 12266–12273 CrossRef CAS.
- C. Chakraborty, P. K. Sukul, K. Dana and S. Malik, Ind. Eng. Chem. Res., 2013, 52, 6722–6730 CrossRef CAS.
- A. Walcarius, Chem. Mater., 2001, 13, 3351–3372 CrossRef CAS.
- K. Das, S. S. Ray, S. Chapple and J. Wesley-Smith, Ind. Eng. Chem. Res., 2013, 52, 6083–6091 CrossRef CAS.
- F. Pan, F. Peng, L. Lu, J. Wang and Z. Jiang, Chem. Eng. Sci., 2008, 63, 1072–1080 CrossRef CAS PubMed.
- Y. Tamai, H. Tanaka and K. Nakanishi, Macromolecules, 1994, 27, 4498–4508 CrossRef CAS.
- Y. Tamai, H. Tanaka and K. Nakanishi, Macromolecules, 1995, 28, 2544–2554 CrossRef CAS.
- Y. Tamai, H. Tanaka and K. Nakanishi, Fluid Phase Equilib., 1995, 104, 363–374 CrossRef CAS.
- M. Heuchel, D. Hofmann and P. Pullumbi, Macromolecules, 2004, 37, 201–214 CrossRef CAS.
- B. Sakintuna, E. Fakioglu and Y. Yȕrȕm, Energy Fuels, 2005, 19, 2219–2224 CrossRef CAS.
- W. Z. Lang, Z. L. Xu and H. Yang, et al., J. Membr. Sci., 2007, 288, 123–131 CrossRef CAS PubMed.
- X. C. Cao, J. Ma and X. H. Shi, et al., Appl. Surf. Sci., 2006, 253, 2003–2010 CrossRef CAS PubMed.
- A. Bottino, G. Capannelli and A. Comite, Desalination, 2002, 146, 35–40 CrossRef CAS.
- Y. Lu, S. L. Yu and B. X. Chai, Polymer, 2005, 46, 7701–7706 CrossRef PubMed.
- D. J. Lin, C. L. Chang and L. P. Cheng, et al., Polymer, 2003, 44, 413–422 CrossRef CAS.
- T. Ogoshi and Y. Chujo, J. Polym. Sci. A: Polym. Chem., 2005, 43, 3543–3550 CrossRef CAS PubMed.
- H. Sun, Macromolecules, 1936, 28, 701–712 CrossRef.
- H. Sun, J. Phys. Chem. B, 1998, 102, 7338–7364 CrossRef CAS.
- S. L. Mayo, B. D. Olafson and W. A. Goddard, J. Phys. Chem., 1990, 94, 8897–8909 CrossRef CAS.
- H. C. Andersen, J. Chem. Phys., 1980, 72, 2384–2393 CrossRef CAS PubMed.
- H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren, A. Dinola and J. R. Haak, J. Chem. Phys., 1984, 81, 3684–3960 CrossRef CAS PubMed.
- P. P. Ewald, Ann. Phys., 1921, 64, 253–287 CrossRef PubMed.
- N. Karasawa and W. A. Goddard, Macromolecules, 1992, 25, 7268–7281 CrossRef CAS.
- H. Wang, L. Yao, B. Ding, J. Luo, G. Zhou and B. Jiang, Chem. J. Chin. Univ., 2013, 34, 1295–1302 CAS.
- L. Yao, P. Chen, B. Ding, J. Luo, B. Jiang and G. Zhou, J. Mol. Model., 2012, 18, 4529–4545 CrossRef CAS PubMed.
- T. T. Moore and W. J. Koros, J. Mol. Struct., 2005, 739, 87–98 CrossRef CAS PubMed.
- R. K. Bharadwaj, R. J. Berry and B. L. Farmer, Polymer, 2000, 41, 7209–7221 CrossRef CAS.
- J. R. Fried and P. Ren, Comput. Theor. Polym. Sci., 2000, 10, 447–463 CrossRef CAS.
- J. R. Fried and D. K. Goya, J. Polym. Sci., Part B: Polym. Phys., 1998, 36, 519–536 CrossRef CAS.
- C. Nagel, E. Schmidtke, K. Günther-Schade, D. Hofmann, D. Fritsch, T. Strunskus and F. Faupel, Macromolecules, 2000, 33, 2242–2248 CrossRef CAS.
- D. Fragiadakis, P. Pissis and L. Bokobza, Polymer, 2005, 46, 6001–6008 CrossRef CAS PubMed.
- F. Peng, L. Lu, H. Sun, Y. Wang, J. Liu and Z. Jiang, Chem. Mater., 2005, 17, 6790–6796 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09261b |
| This journal is © The Royal Society of Chemistry 2015 |