Robust spin-on-glass poly(methyl)silsesquioxane-based low-k materials derived from a cyclic siloxane precursor†
Albert S. Leea,
Sung Yeoun Oha,
Seung-Sock Choia,
He Seung Leea,
Seung Sang Hwangab and
Kyung-Youl Baek*ab
aMaterials Architecturing Research Center, Korea Institute of Science and Technology, Hwarangno 14-gil 5, Seong-Buk Gu, Seoul 136-791, Republic of Korea. E-mail: baek@kist.re.kr
bNanomaterials Science and Engineering, University of Science and Technology, 217 Gajungro, 176 Gajung-dong, Yuseong-Gu, Daejeon 305-333, Republic of Korea
First published on 29th July 2015
Abstract
A series of organic–inorganic hybrid spin-on-glass polymethylsilsesquioxanes were synthesized utilizing a cyclic siloxane precursor, 1,3,5,7-tetramethyl-1,3,5,7-tetrahydroxyl cyclosiloxane (MT4-OH), copolymerized with methyltriethoxysilane (MTES) at various comonomer ratios. By selectively introducing this 2-D cyclic crosslinker, we were able to obtain spin-on-glass hybrimers with low dielectric constant (2.5–2.7), high nanoindentation modulus (5–10.5 GPa), with high thermal stability (>700 °C) without the use of porogens or additives. The use of the cyclic monomer MT4-OH greatly increased the mechanical properties, which allowed for impeccable reliability of a variety of patterns obtained through etching and chemical mechanical planarization processes, while maintaining optimal gap-filling properties. Due to the superior dielectric, mechanical, and integrated processing of these materials, these hybrids derived from MT4-OH may be utilized as next generation spin-on-glass low-dielectric constant materials.
Introduction
Since the microchip technology boom started in the 1980's, the search for low-dielectric constant materials has been extensive and pervasive within both industry and academia to fulfil the need for smaller feature sizes of technology nodes, while reducing signal delays and crosstalk noise between the metal interconnects within integrated circuits.1,2 Moore's law, which has dictated that the integration density of such circuits should double every 12 to 18 months, has led to new challenges from the perspective of a material scientist to overcome, one of which is the development of low-dielectric constant materials.3,4Low-dielectric constant materials must possess a vast myriad of electrical, thermal, chemical, and mechanical properties, which are just as crucial as the low dielectric permittivity property that classifies them.5 In order for low-dielectric constant materials to be successfully integrated into integrated circuits, the material must be able to withstand 10–15 repeated temperature treatments exceeding 400 °C, have low coefficients of thermal expansion (CTE), excellent adhesion to metal interconnects such as copper, chemical resistant to the solvents and etchants commonly used during chip fabrication, as well as superior mechanical properties to withstand the etching processes known as chemical mechanical polishing (CMP).6 As such, organic polymers such as polynorbornenes, polyimides, and poly(aryl ethers), while possessing low-dielectric properties,7 but lacking in one or more of the other prerequisites for actual applications into integrated circuits, have been more or less passed over nowadays.
Polysilsesquioxanes (PSSQs) are a class of inorganic–organic hybrid materials with a single organic functional group covalently linked to a trifunctional silane.8 PSSQs are synthesized via sol–gel processing consisting of hydrolysis of either alkoxy- or chloro-ligands, followed by polycondensation of the silanol groups to form Si–O–Si bridges.8,9 In addition to the superior thermal and mechanical properties imbued by the inorganic Si–O–Si backbones, the organic groups allow for a myriad of applications which require organic functionalities. Moreover, as polysilsesquioxanes are soluble in organic solvents, spin-on-glass PSSQs have been sought out as one of the materials of choice to supplant expensive and time-consuming deposition processes of hybrid films.10
With regards to low-dielectric constant materials, polymethylsilsesquioxanes (PMSSQs) has been the material most extensively researched, due to the low polarizability of the Si–C methyl group, giving rise to its low dielectric constant (∼2.7), while exhibiting superior thermal stabilities (>500 °C).11,12 Numerous studies have detailed the various structure–property relationships of PMSSQs and the different synthetic variables which govern its molecular weight, thermal stability, structure, and their effect on their electrical properties.13 And while numerous studies have investigated the effect of sacrificial porogens to further decrease the dielectric constant,14 issues arising from metal infiltration into pores,15 as well as decrease in mechanical properties were problems left to address in such cases.
More recently, various chemical modifications to the Si–O based hybrids have been reported as new methodologies for new interlayer low-dielectric constant materials. Several groups have reported on the low-dielectric properties of benzoxazine-functionalized polysilsesquioxanes,16,17 cyanate ester composites,18 as well as the functionalization of benzocyclobutene19 to the Si–O–Si backbone to utilize the high free-volume of the crosslinked organic components to substantially lower the dielectric constants. However, despite the improvements in thermal, mechanical, and electrical properties, none of these studies have shown that integration was possible with these new materials.
In this study, we investigated the effect of incorporating Si–C methyl groups via a cyclic siloxane precursor, tetrahydroxyl tetramethyl cyclosiloxane (MT4-OH), copolymerized with methyltriethoxysilane (MTES) at various comonomer ratios. By chemically incorporating this 2D cyclic monomer into the poly(methyl)silsesquioxane matrix, we sought to increase the internal free volume to further decrease the dielectric constant, while examining the thermal, mechanical, electrical, and most importantly, integration reliability of these robust hybrid materials as low-dielectric constant spin-on-glass resins without the use of additives or porogens which may depreciate mechanical properties.
Experimental
Materials
Methyltriethoxysilane (MTES) (Shin Etsu, 99%), 1,3,5,7-tetramethyl-2,4,6,8-tetrahydrido cyclosiloxane (MD4-H) (Gelest, 98%), and tetrahydrofuran (J. T. Baker, HPLC grade) were vacuum distilled prior to use. Pd/C 10% was purchased from Acros. Deionized water was used for hydrolysis of MD4-H and sol–gel reaction with MT4-OH and MTES. All other solvents were used as received.Characterization
Weight averaged molecular weight (Mw) and molecular weight distributions (Mw/Mn) of the polymers were measured by JASCO PU-2080 plus SEC system equipped with refractive index detector (RI-2031 plus), UV detector (λ = 254 nm, UV-2075 plus), and Viscotek SLS apparatus using THF as the mobile phase at 40 °C with a flow rate of 1 ml min−1. The samples were separated through four columns (Shodex-GPC KF-802, KF-803, KF-804, KF-805). 1H-NMR, 29Si NMR spectra were recorded in CDCl3 at 25 °C on a Varian Unity INOVA (1H: 300 MHz, 29Si: 59.6 MHz). FT-IR spectra were measured using Perkin-Elmer FT-IR system (Spectrum-GX) using solvent cast films on KBr pellets. Thermal gravimetric analysis (TGA) was performed by TA Instrument TGA 2950 under N2. Nanoindentation measurements were conducted on a Hysitron Inc. TriboIndenter equipped with a Berkovich diamond tip. Measurements of modulus were performed as a continuous stiffness measurement on samples coated on silicon wafers at thickness of 100 nm. Dielectric constants were measured using metal–insulator–metal (MIM) method with thermally evaporated aluminum as metal with PMSSQ resins spin coated with 25 wt% propylene glycol monomethyl ether acetate (PGMEA) slurries with thickness ∼500 nm. All integration tests were conducted at the KAIST Nanofab Center.Synthesis of 1,3,5,7-tetramethyl 1,3,5,7-tetrahydroxyl cyclosiloxane (MT4-OH)
Hydrolysis of the hydride, 1,3,5,7-tetramethyl-2,4,6,8-tetrahydrido cyclosiloxane (MD4-H) (19 ml, 0.089 mol) was carried out with water (6.4 ml, 0.36 mol) and 10% activated Pd/C (0.8 g) for 2 h in THF (220 ml) at 10 °C, according to Scheme 1. Excess water and Pd/C were removed by dispersing the reaction solution in MgSO4 and cellite, followed by filtering and slowly evaporating excess THF at temperatures below 20 °C. Obtained MT4-OH was stored as a powder at −15 °C prior to use.Typical synthesis of MT4-MSSQ
In a 3-necked jacketed flask set at 25 °C, a reaction solution of H2O (2.4 g, 0.13 mol), THF (20 ml), and concentrated HCl (1.23 g) was first prepared. To this reaction mixture, a total silane monomer mixture (0.04 mol) was added dropwise over 10 minutes. After addition of the silane monomers, the reaction flask was sealed and magnetically stirred for 24 h at 25 °C, and then for 19 h at 35 °C. After upon completion of the hydrolysis–polycondensation reactions, the reaction mixture was dissolved in dichloromethane and extracted with water several times to remove HCl. Drying over MgSO4, filtering, and evaporating dichloromethane at temperatures below 20 °C, MT4-MSSQ resins were obtained as white powders, which were stored at −15 °C. PMSSQ resins were named as such: MT4-MSSQ37 for SOG resins synthesized with MT4-OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTES mol ratio of 3
MTES mol ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.
7.
Results and discussion
Synthesis and characterization of MT4-MSSQ spin on glass resins
The synthesis of 1,3,5,7-tetramethyl 1,3,5,7-tetrahydroxyl cyclosiloxane (MT4-OH) was carried out following our previous method,20 through quantitative hydrolysis of 1,3,5,7-tetramethyl-2,4,6,8-tetrahydrido cyclosiloxane (MD4-H) with water, catalyzed with Pd/C at low temperatures. The obtained MT4-OH precursor was then utilized as sol–gel comonomer with methyltriethoxysilane, as our previous studies using only MT4-OH yielded ladder-like structures.20,21 As our objective was to synthesize a set of thermally curable PMSSQs with random branched structures containing Si–OH groups, we hypothesized that the PMSSQ network incorporating the MT4-OH monomer would improve upon the mechanical properties of the cured final film, due to the inherently condensed Si–O–Si half cage structure of MT4-OH.The synthesis of MT4-MSSQs was straightforward, as we utilized a modified literature procedure for which MTES-based sol–gel conditions were well-known.13 Moreover, this acid-catalyzed sol–gel reaction allowed for fast and complete hydrolysis of the ethoxy groups of MTES. The MT4-MSSQs were named according to the monomer mol ratio of MTES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MT4-OH followed the MT4-MSSQ code as such: for MTES
MT4-OH followed the MT4-MSSQ code as such: for MTES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MT4-OH mol ratio of 3
MT4-OH mol ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, the MT4-MSSQs were named MT4-MSSQ37. In this study, we studied the various spin-on-glass PMSSQs with MTES
7, the MT4-MSSQs were named MT4-MSSQ37. In this study, we studied the various spin-on-glass PMSSQs with MTES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MT4-OH mol ratio of 0.5
MT4-OH mol ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.5, 1
9.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 2
9, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 3
8, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, and 5
7, and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Increasing the MT4-OH ratio over 50% resulted in a precipitous loss in solubility and were excluded for further study.
5. Increasing the MT4-OH ratio over 50% resulted in a precipitous loss in solubility and were excluded for further study.
The obtained MT4-MSSQ spin-on-glass resins were first characterized by 1H NMR and FTIR. As shown in Fig. 1a, the ethoxy groups of MTES for the obtained MT4-MSSQs were completely hydrolysed as indicated by the lack of ethoxy groups at around 3.8 ppm. This complete hydrolysis of alkoxy groups has been reported as a critical point for spin-on-glass low dielectric constant materials, as unhydrolyzed alkoxy groups around contribute to substantial film shrinkage, compared to their hydrolyzed Si–OH silanol groups when thermally cured under high temperatures.13,22 While small amounts of silanol groups were found at 7.3 ppm, the relative amounts were difficult to quantify due to the weak and broad signals. In addition, the FTIR spectrum for the various MT4-MSSQs (Fig. 1b) revealed that all the PMSSQ resins were of high molecular weight and random-branched structure, as indicated by the sharp and doubly split Si–O–Si peaks ranging from 1000–1200 cm−1 characterized as a and b.8,13 Moreover, the peaks centered at 960 cm−1 and 3500 cm−1 were assigned to the uncondensed Si–OH silanol groups, c. Interestingly, the relative amounts of uncondensed Si–OH silanol groups slightly increased as MTES content increased, which was surprising considering the amorphous networking of the Si–O–Si bonds during the hydrolysis–polycondensation reaction leading to similar degrees of condensation under acidic conditions.13 However, as shown, as MTES content increased, uncondensed silanol content increased slightly, indicating that the MT4-OH monomer functioned to increase the degree of condensation due the tetrasiloxane core only required polycondensation reaction between silanols to obtain highly condensed rigid Si–O–Si networks.
In order to elucidate the optimal curing temperature for the MT4-MSSQ resins, thermal curing was carried out 100 °C, 200 °C, and 400 °C for 2 h for the MT4-MSSQ37 spin-on-glass resin. As shown, the silanol peaks c, completely disappeared after thermal curing, indicating that the thermal curing process was complete, even at 100 °C. However, for conventional low-dielectric constant materials, incomplete thermal curing is a critical issue, as uncondensed silanol groups contribute to both diffusion of metal, compromising the metal–dielectric interface, and in some cases failing to insulate the metal inconnects.5 As such, high temperature curing at 200 °C and 400 °C were investigated. As expected, uncured silanol groups were not detected, but interestingly, the relative intensity of the Si–O–Si bonding peaks around 1000–1200 cm−1 changed. This was attributed to the change in Si–O–Si networking after thermal curing providing a highly crosslinked internal Si–O–Si network when cured at 400 °C.
Additionally, the SEC derived weight averaged molecular weights (Mw) for all of the MT4-MSSQ spin-on-glass resins were tabulated in Table 1. As shown, the Mw values for all MT4-MSSQ resins were in the range of 10–14k, with Mw increasing as MT4-OH mol ratio increased. The increase in Mw was attributed to the innate characteristic of the four silanol groups of MT4-OH, acting as branching points for intramolecular and intermolecular condensations between MT4-OH and MTES. Also, the polydispersity indices (PDI) for all of the MT4-OH resins were between 2.2 and 2.5, indicating the stabilization of network structure as obtained sols.
| Sample name | Mn | Mw | PDI |
|---|---|---|---|
| MT4-MSSQ595 | 4700 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2.2 |
| MT4-MSSQ19 | 4600 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.3 |
| MT4-MSSQ28 | 4500 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2.5 |
| MT4-MSSQ37 | 5000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2.4 |
| MT4-MSSQ55 | 5900 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
2.3 |
The degree of condensation (DoC) of the Si–O–Si bonds was investigated by 29Si NMR. As shown in Fig. 3, two characteristic peaks were observed and characterized to the T3 alkyl-Si(OSi–)3 and T2 alkyl-Si(OSi–)2OH peaks centered at −68 ppm and −58 ppm, respectively.20,23 The degree of condensation as described by Bae,24,25 is the degree in which all of the Si–O silicons are condensed, respective to the uncondensed silicons. Thus, for purely T resins, the degree of condensation (DoC) can be simply expressed by the integrated ratio of T3 to T2 characteristic peaks. In the MT4-MSSQ spin-on-glass resins obtained in this study, all of the degree of condensation values were about 75–92%, indicating that the Si–O–Si frameworks were mostly condensed. This is an important feature, as excess uncondensed Si–OH groups contribute to shrinkage of the coating layer induced by the thermal treatments required for curing,13,22 which invariably leads to both a decrease in mechanical properties and interface reliability. As the MT4-OH contents increased, a slight increase in degree of condensation was observed, most likely due to the MTES monomers acting as branching points and probably source of uncondensed silanol groups.
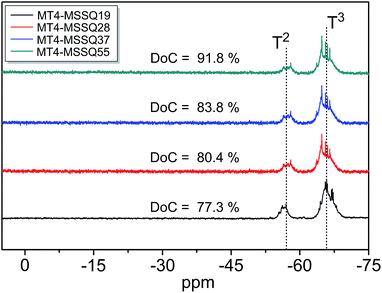 | ||
| Fig. 3 29Si NMR spectra for MT4-MSSQ spin-on-glass resins with calculated degrees of condensation (DoC) values. | ||
Thermal, mechanical, and electrical properties
Thermal stability is considered to be one of the most important parameters in evaluating perspective materials for low-dielectric constant applications due to the numerous thermal treatments which are required for chip fabrication.1–5 Fig. 4 exhibits the thermogravimetric analysis (TGA) results for all of the MT4-MSSQ spin-on-glass resins. The MT4-MSSQ spin-on-glass resins exhibited exceptional thermal stability, with about 15–10 wt% loss attributed to the secondary condensations between the uncondensed Si–OH groups at 150 °C and less than 3 wt% loss between temperatures 300 °C to 700 °C. While the thermal stability of the MT4-MSSQ resins were very similar, slight differences in the thermal degradation starting at 150 °C attributed to the uncondensed silanol groups were observed, as this initial relative thermal degradation increased as MTES content increased, representative of the DoC values calculated from the 29Si NMR spectra in Fig. 3.Next, the dielectric properties for the MT4-MSSQ spin-on-glass resins were examined. In Fig. 5, the dielectric constant and breakdown strength values for various MT4-MSSQ spin-on-glass resins after curing were shown. The breakdown voltage values for all of the MT4-MSSQ spin-on-glass resins all exceeded 2.0 MV cm−1 and remained relatively the same regardless of comonomer composition. However, the dielectric constant values increased from 2.2 to 2.75 with increase in MT4-OH contents. While this was contrary to what we hypothesized, the increase in dielectric constant was attributed to the relatively denser internal Si–O–Si structure formed through the polycondensations between MT4-OH monomers within the MT4-MSSQ network. Moreover, the MTES monomer most likely acted as branching and or curing point as uncondensed silanol groups were primarily derived from MTES, as indicated by the lower degree of condensations for MT4-MSSQ resins containing higher MTES content. We were also able to discern that once the MT4-OH contents increased over 30%, the dielectric constant values reached a plateau around 2.75, which is more or less the theoretical value for poly(methyl)silsesquioxanes (PMSSQs),1 and also very close to the dielectric constant of the MT4-OH monomer itself (k = 3.0). Moreover, when the dielectric constant values were compared to the non-cured, as-cast thin films shown in ESI, Fig S1,† the dielectric constants were approximately 0.2 lower, indicative of the full curing of silanol groups, also supported by FTIR results in Fig. 2.
 | ||
| Fig. 5 Dielectric constant and breakdown voltage values for MT4-MSSQ spin-on-glass resins after curing. | ||
Mechanical properties of the MT4-MSSQ spin-on-glass resins were evaluated using nanoindentation method.26,27 As shown in Fig. 6 and detailed in Table 2, the elastic moduli and hardness values increased as MT4-OH contents increased, with MT4-MSSQ55 giving an elastic modulus of 10.5 GPa and hardness of 1.5 GPa. In contrast to the non-cured, as-cast MT4-MSSQ resins, the elastic modulus values were approximately 2–3 GPa greater, and the hardness values 0.5–1 GPa greater as shown in ESI, Fig S2.† This was in line with our previous discussion of the MT4-OH providing internally dense and mechanically robust inorganic backbones. This point was further supplanted by observation of the brittleness index,27 commonly defined by the hardness divided by the elastic modulus. As tabulated in Table 2, the brittleness index for MT4-MSSQ resins after thermal curing decreased with increasing MT4-OH contents, suggesting that the MTES monomers giving rise to uncondensed silanols, as indicated by the relatively lower degree of condensations in Fig. 2 and 3, provided a greater degree of crosslinking between sol–gel matrices.
| Sample name | Elastic modulus, E (GPa) | Hardness, H (GPa) | Brittleness index (H/E) | Plasticity index (E/H) |
|---|---|---|---|---|
| MT4-MSSQ595 | 4.8 | 0.75 | 0.156 | 6.40 |
| MT4-MSSQ19 | 5.0 | 0.77 | 0.154 | 6.49 |
| MT4-MSSQ28 | 6.1 | 0.89 | 0.146 | 6.85 |
| MT4-MSSQ37 | 9.0 | 1.29 | 0.144 | 6.98 |
| MT4-MSSQ55 | 10.5 | 1.5 | 0.143 | 7.00 |
Integration tests with MT4-MSSQ37
For the integration tests, we chose MT4-MSSQ37 as our testing sample, due to the best balance in electrical and mechanical properties. First, the coating properties on Si wafers was confirmed by spin coating the 25 wt% MT4-MSSQ37 slurry in PGMEA, and the 3D confocal microscope and SEM image shown in Fig. 7a as impeccable, smooth film. After curing, the resulting film was wet-etched in a 5 wt% HF aqueous solution for 30 min under ambient conditions. After etching, dissolution of the MT4-MSSQ37 film was shown in Fig. 7b and a substantial increase in roughness was observed. However, the surface was intact proving the chemical stability of the MT4-MSSQ37 resin towards harsh acidic conditions. After chemical mechanical polishing (CMP) process, a smooth and pure film was recovered. This recovery of the initial smooth surface revealed not only that the MT4-MSSQ37 resin possessed the necessary etching capability required of siloxane-based low-dielectric constant maters, but also retained the prerequisite mechanical properties (surface modulus and hardness) for one for the most basic semiconductor processing tests.In addition to testing the reliability of the MT4-MSSQ37 resins against CMP processes, metal–insulator and metal–insulator–metal (MIM) structures were fabricated as shown in Fig. 8. As shown, the MT4-MSSQ37 resin possesses excellent adhesion to aluminum and the patterned Si wafer, as shown in Fig. 8a, without noticeable delamination, cracks, pinholes, or defects. In addition, Fig. 8b revealed the MIM structure with MT4-MSSQ37 sandwiched between aluminum and tin electrodes. As shown, the interface between both aluminum and tin electrodes were impeccable, with the diffusion barrier thickness around 100 nm and MT4-MSSQ37 resins both having unblemished surface interfaces, due to the excellent processability of MT4-MSSQ37, as well as the thermal and mechanical stability to withstand the metal deposition processes.
Finally, the gap-filling properties of MT4-MSSQ37 coated on patterned Si wafers was investigated. In Fig. 9, the SEM images of MT4-MSSQ37 coated surfaces with various aspect ratios showed that the MT4-MSSQ37 fully filled the etched trenches of the patterned Si wafers while providing a smooth coated interface on top of the Si wafer, again showing the utility of the excellent processability and coating properties of MT4-MSSQ37 spin-on-glass resins.
 | ||
Fig. 9 SEM image of MT4-MSSQ coated on pattered Si wafers with (a) aspect ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and (b) aspect ratio of 3 10 and (b) aspect ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 at various magnifications. 4 at various magnifications. | ||
Conclusion
In summary, a series of poly(methyl)silsesquioxane spin-on-glass resins were synthesized and characterized utilizing cyclic monomer copolymerized with methyltriethoxysilane. Various thermal, mechanical, and electrical properties of these PMSSQ resins were explored for low-dielectric constant materials and were found to be well within the acceptable range for integration tests. Integration tests with the MT4-MSSQ37 resin revealed excellent processability and mechanical properties to withstand CMP processes, superb adhesion to metal, and good gap-filling properties.Acknowledgements
This work was financially supported by a grant from the Fundamental R&D Program for Core Technology of Materials funded by the Ministry of Knowledge, Economy, Republic of Korea and partially by the International Collaborative R&D Program (N0000678) funded by the Ministry of Trade, Industry, and Energy, (MOTIE, Korea).Notes and references
- W. Volksen, R. D. Milller and G. Dubois, Chem. Rev., 2010, 110, 56 CrossRef CAS PubMed.
- G. Dubois, W. Volksen, T. Magbittang, R. D. Miller, D. M. Gage and R. H. Dauskardt, Adv. Mater., 2007, 19, 3989 CrossRef CAS PubMed.
- T. Frot, W. Volksen, S. Purushothaman, R. Bruce and G. Dubois, Adv. Mater., 2011, 23, 2828 CrossRef CAS PubMed.
- T. Jiang, B. Zhu, S.-J. Ding, Z. Fan and D. W. Zhang, J. Mater. Chem. C, 2014, 2, 6502 RSC.
- S. W. King, D. Jacob, D. Vanleuven, B. Colvin, J. Kelly, M. French, J. Bielfeld, D. Dutta, M. Liu and D. Gidley, ECS J. Solid State Sci. Technol., 2012, 1, 115 CrossRef PubMed.
- M. Krishnan, J. W. Nalaskowski and L. M. Cook, Chem. Rev., 2010, 110, 178 CrossRef CAS PubMed.
- G. Zhao, T. Ishizaka, H. Kasai, M. Hasegawa, T. Furukawa, H. Nakanishi and H. Oikawa, Chem. Mater., 2009, 21, 419 CrossRef CAS.
- R. H. Baney, M. Itoh, A. Sakakibara and T. Suzuki, Chem. Rev., 1995, 95, 1409 CrossRef CAS.
- D. Loy and K. J. Shea, Chem. Rev., 1995, 95, 1431 CrossRef CAS.
- B. Lee, Y.-H. Park, Y.-T. Hwang, W. Oh, J. Yoon and M. Rhee, Nat. Mater., 2005, 4, 147 CrossRef CAS PubMed.
- L.-H. Lee, W.-C. Chen and W.-C. Liu, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 1560 CrossRef CAS PubMed.
- C.-S. Kim and H.-D. Jeong, J. Phys. Chem. B, 2008, 112, 16257 CrossRef CAS.
- H. W. Ro, E. S. Park, C. L. Soles and D. Y. Yoon, Chem. Mater., 2010, 22, 1330 CrossRef CAS.
- J. Abdallah, M. Silver, S. A. B. Allen and P. A. Koul, J. Mater. Chem., 2007, 17, 873 RSC.
- T. M. Hermans, J. Choi, B. G. G. Lohmeijer, G. Dubois, R. C. Pratt, H.-C. Kim, R. M. Waymouth and J. L. Hedrick, Angew. Chem., Int. Ed., 2006, 45, 6648 CrossRef CAS PubMed.
- R. Sasi kumar and M. Alagar, RSC Adv., 2015, 5, 33008 RSC.
- R. Sasi kumar, N. Padmanathan and M. Alagar, New J. Chem., 2015, 39, 3995 RSC.
- S. Devaraju, M. R. Vengatesan, M. Selvi, A. A. Kumar, I. Hamerton, J. S. Go and M. Algar, RSC Adv., 2013, 3, 12915 RSC.
- L. Kong, Y. Cheng, Y. Jin, Z. Ren, Y. Li and F. Xiao, J. Mater. Chem. C, 2015, 3, 3364 RSC.
- H. S. Lee, S.-S. Choi, K.-Y. Baek, S. M. Hong, E. C. Lee, J.-C. Lee and S. S. Hwang, Eur. Polym. J., 2012, 48, 1073 CrossRef CAS PubMed.
- H. S. Lee, S.-S. Choi, K.-Y. Baek, S. M. Hong, E. C. Lee, J.-C. Lee and S. S. Hwang, Macromol. Res., 2012, 20, 1131 CrossRef CAS.
- H.-J. Lee, E. K. Lin, H. Wang, W.-L. Wu, W. Chen and E. S. Moyer, Chem. Mater., 2002, 14, 1845 CrossRef CAS.
- S.-S. Choi, A. S. Lee, H. S. Lee, H. Y. Jeon, K.-Y. Baek, D. H. Choi and S. S. Hwang, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 5012 CrossRef CAS PubMed.
- J. S. Kim, S. C. Yang and B. S. Bae, Chem. Mater., 2010, 22, 3549 CrossRef CAS.
- S. C. Yang, J. H. Jin, S. Y. Kwak and B. S. Bae, J. Appl. Polym. Sci., 2012, 126, 380 CrossRef PubMed.
- A. M. Diez-Pascual, M. A. Gomez-Fatou, F. Ania and A. Flores, Prog. Mater. Sci., 2015, 67, 1 CrossRef CAS PubMed.
- X. Zhang, L. Hu and D. Sun, Acta Mater., 2006, 54, 5469 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Dielectric constant values and mechanical properties of as-cast MT4-OH resins. See DOI: 10.1039/c5ra11110b |
| This journal is © The Royal Society of Chemistry 2015 |







