An effective quaternary nano-sized Er3+:Y3Al5O12/Pt–PdS/ZnS visible-light photocatalyst for H2 production
Chunxiao
Lu
a,
Yang
Chen
a,
Yun
Li
a,
Chunhong
Ma
a,
Hongbo
Zhang
a,
Yuwei
Guo
ab and
Jun
Wang
*a
aCollege of Chemistry, Liaoning University, Shenyang 110036, P.R. China. E-mail: wangjun888tg@126.com; wangjun891@sina.com; Fax: +86 24 62202053; Tel: +86 24 62207861
bDepartment of Chemistry, Baotou Normal College, Baotou 014030, P.R. China
First published on 15th June 2015
Abstract
A highly effective and stable up-conversion luminescence agent, Er3+:Y3Al5O12, was synthesized by sol–gel and calcination methods. Then, as a novel visible-light photocatalyst, Er3+:Y3Al5O12, Pt and PdS (as dual co-catalysts) decorated ZnS nano-sized composites, Er3+:Y3Al5O12/Pt–PdS/ZnS, were fabricated by deposition–precipitation and ultrasonic dispersion methods. All prepared samples were characterized by X-ray diffractometry (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS) and energy dispersive X-ray spectroscopy (EDX). UV-vis absorption and PL spectra of Er3+:Y3Al5O12 were also determined. The visible-light photocatalytic H2 production activity of the prepared Er3+:Y3Al5O12/Pt–PdS/ZnS was evaluated by using Na2S and Na2SO3 as sacrificial reagents in an aqueous solution under 300 W irradiation from a xenon lamp. In addition, some influential factors such as Er3+:Y3Al5O12 and ZnS mass ratio, catalyst amount, irradiation time and irradiation intensity on visible-light photocatalytic H2 production of Er3+:Y3Al5O12/Pt–PdS/ZnS were investigated in detail. It was found that Er3+:Y3Al5O12 can effectively improve the visible-light photocatalytic activity of ZnS. Particularly, Er3+:Y3Al5O12/Pt–PdS/ZnS with 0.13 wt% PdS and 0.3 wt% Pt and a 0.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 mass ratio shows the highest photocatalytic H2 production activity in the sulfide/sulfite (0.2 mol L−1 Na2S and 0.3 mol L−1 Na2SO3) aqueous solution as sacrificial reagents.
1.00 mass ratio shows the highest photocatalytic H2 production activity in the sulfide/sulfite (0.2 mol L−1 Na2S and 0.3 mol L−1 Na2SO3) aqueous solution as sacrificial reagents.
1. Introduction
With increasing human activities, fossil fuels, which have caused many serious environmental problems, are struggling to maintain the growing energy demand. Therefore, a clean and sustainable energy of the future has aroused extensive attention. Hydrogen, the cleanest fuel, is expected to surpass fossil fuels.1–4 In particular, photocatalytic H2 production from water splitting using semiconductor photocatalysts has drawn considerable attention as a promising way of resolving energy and environmental problems.5–7Since the Honda–Fujishima effect was first reported,8 many kinds of photocatalysts for the water decomposition have been developed by many researchers. Recently, metal sulfides have been intensively studied as active photocatalysts due to their unique catalytic functions.9–13 Among them, the wide band gap (ca. 3.6 eV) of ZnS photocatalyst exhibits rapid generation of electron–hole pairs upon photoexcitation and highly negative reduction potentials of excited electrons. However, owing to relatively wide band gap, it can be activated mainly under ultraviolet-light which accounts for less than 5.0% of the solar light energy reaching the Earth's surface and therefore imposes restrictions on the application of ZnS photocatalysts.14–16 To obtain higher activity for photocatalytic H2 production from water splitting by ZnS, much more techniques have been used, for example, addition of sacrificial agents,17,18 surface modification of photocatalysts, doping of metal or nonmetal ions,19,20 semiconductor coupling.21,22 These methods are proved to be effective, but, they reduce the catalytic activity of ZnS itself. It is the best way to maintain the catalytic activity of the wide band gap of ZnS and meet the energy needs. Recently, in our researches a new improved method was proposed, that is, some photocatalysts are combined with up-conversion luminescence agent to improve photocatalytic H2 production from water splitting.
We emphasize a solely photonics approach to enhance the photocatalytic activity: a blue shift by up-conversion luminescence agent with the ability to transform visible-light into ultraviolet-light, which satisfy the requirements of large band-gap semiconductor materials. During the past decades, up-conversion luminescence agent, which can convert longer wavelength radiation to shorter wavelength fluorescence via a two-photon or multi-photon mechanism, have attracted a tremendous amount of attention, for example, Yb3+/Er3+:NaGdF4, Yb3+/Er3+:YVO4 and Er3+:Y3Al5O12etc., have been reported.23–25 Among them, Er3+:Y3Al5O12 was deemed to be a most efficient visible-to-ultraviolet up-conversion luminescence agent, which can effectively activate the ZnS to carry out visible-light photocatalytic reaction.
So far, the efficiency of photocatalytic H2 production from water splitting is still low. A key reason is that the photogenerated electrons/holes are easily consumed via recombination. In order to promote the rapid surface transfer of photogenerated electrons and holes from photocatalyst, the widely used strategy for achieving this purpose is loading co-catalysts. During the past few years, various co-catalysts have been reported to be utilized on photocatalyst, such as Pt, MoS2, RuO2 and PdS, whereas the role of the co-catalysts in the reaction is not the same. Pt and MoS2 are effective reduction co-catalysts (RCs), while PdS and RuO2 are effective oxidation cocatalysts (OCs). They are proved to be beneficial for the efficient charge separation and surface reactions, which achieve a higher efficiency of photocatalytic H2 production from water splitting. So, the use of dual co-catalysts may be crucial for improve the photocatalytic activity.26–30
Herein, an effective and stable up-conversion luminescence agent, Er3+:Y3Al5O12, was synthesized by sol–gel method and the nano-sized ZnS particles were obtained by hydrothermal method. And then, a novel visible-light photocatalyst, Er3+:Y3Al5O12/Pt–PdS/ZnS, was successfully prepared by deposition and calcination methods. The experimental results showed that the photocatalytic H2 production activity of ZnS could be significantly enhanced by the presence of Er3+:Y3Al5O12 and by supported Pt and PdS as dual co-catalyst. How the Er3+:Y3Al5O12 affects the prepared nano-sized Pt–PdS/ZnS particles for visible-light photocatalytic H2 production activity was also investigated in detail. In addition, the mechanism of up conversion luminescence process of Er3+:Y3Al5O12 and the excitation principle of Er3+:Y3Al5O12/Pt–PdS/ZnS photocatalysts under visible-light irradiation were proposed.
2. Experimental procedure
2.1. Materials and reagents
Erbium oxide (Er2O3, 99.999%), yttrium oxide (Y2O3, 99.999%) and aluminum nitrate nonahydrate ((AlNO3)3·9H2O, analytical pure), citric acid (C6H8O7, analytical pure) and nitric acid (HNO3, 65%, analytical pure, Veking Company, China) were used to synthesize the up-conversion luminescence agent (Er3+:Y3Al5O12). Zinc acetate dihydrate (C4H6O4Zn·2H2O, 99%, Sinopharm Chemical Reagent Co., Ltd, China), dodecyl sodium sulfate (C12H25NaO4S, chemically pure, Sinopharm Chemical Reagent Co., Ltd, China) and sodium sulfide nonahydrate (Na2S·9H2O, 98%, Aladdin Industrial Corporation, China) were used to synthesize the nano-sized ZnS particles. Hexachloroplatinic acid hexahydrate (H2PtCl6·6H2O, 97%, Sinopharm Chemical Regent Co., Ltd, China) and palladium chloride (PdCl2, analytical pure, Sinopharm Chemical Reagent Co., Ltd, China) were used as dual co-catalysts precursor. Sodium sulfite anhydrous (Na2SO3, 97%, Sinopharm Chemical Regent Co., Ltd, China) and sodium sulfide nonahydrate (Na2S·9H2O, 98%, Sinopharm Chemical Regent Co., Ltd, China) were dissolved with double distilled water (Millipore Corporation, USA) as sacrificial agent. All chemicals were used without further purification.2.2. Synthesis of up-conversion luminescence agent (Er3+:Y3Al5O12)
The up-conversion luminescence agent, Er3+:Y3Al5O12, was synthesized by a nitrate–citrate sol–gel and calcination ways.31,32 At the beginning, Y(NO3)3 and Er(NO3)3 solutions were planted by dissolving Y2O3 (0.01 mol, 2.2715 g) and Er2O3 (3.34 × 10−5 mol, 0.0128 g) into suitable hot HNO3 solution (about 60 °C), respectively. The planted Y(NO3)3 and Er(NO3)3 solutions were put together with Al(NO3)3·9H2O (0.0336 mol, 12.6208 g) while stirring magnetically, and the homogenous solution was acquired. Then, the solid citric acid (0.1680 mol, 33.9351 g) was also put into the mentioned mixture solution (mol ratio of citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal ion is 3
metal ion is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The solution was continued to be stirred and heated at 50–60 °C until the transparent sol was triumphantly come into being. And then, the transparent sol was heated at 80 °C for 24 h and became the gel. After the gel cooled in the air, it was ground into good homogeneous powders. For the sake of removing residual organic components and nitrate ions the powders were heated at 1100 °C for 2.0 h in a muffle furnace. In the end, the sintered substance was removed and permitted to cool down to the room temperature in atmosphere. Whereupon, the white Er3+:Y3Al5O12 particles were acquired.
1). The solution was continued to be stirred and heated at 50–60 °C until the transparent sol was triumphantly come into being. And then, the transparent sol was heated at 80 °C for 24 h and became the gel. After the gel cooled in the air, it was ground into good homogeneous powders. For the sake of removing residual organic components and nitrate ions the powders were heated at 1100 °C for 2.0 h in a muffle furnace. In the end, the sintered substance was removed and permitted to cool down to the room temperature in atmosphere. Whereupon, the white Er3+:Y3Al5O12 particles were acquired.
2.3. Preparation of visible-light photocatalyst (Er3+:Y3Al5O12/Pt–PdS/ZnS)
ZnS nanospheres were prepared using hydrothermal method.33–35 At first, sodium dodecylsulfate (0.3 g) was added to the distilled water (25 mL) to form a solution. Zn(acac)2 (1.76 g) was dissolved in the above solution slowly. Subsequently, Na2S (0.4 mol L−1, 20 mL) was added dropwise to the mixture under stirring magnetically for 60 min, and then added different mass ratios of the above prepared Er3+:Y3Al5O12. The as-formed solution was transferred into Teflon lined stainless steel autoclave (100 mL) and maintained at 160 °C for 2.0 h. After cooling to room temperature naturally, a white suspension was separated by centrifugation, washed with distilled water and ethanol several times, and then dried at 60 °C in a vacuum.Modification of Er3+:Y3Al5O12/ZnS with PdS (0.13 wt%) and Pt (0.30 wt%) as dual co-catalysts was realized by deposition–precipitation of PdS and then ultrasonic dispersion and liquid boiling method of Pt.36–38 The photocatalysts were prepared by following two procedures: firstly, a PdCl2 aqueous solution (0.0011 mol L−1) was added dropwise to a suspension of Er3+:Y3Al5O12/ZnS powder (1.0 g, as prepared above) dispersed in Na2S aqueous solution (0.5 mol L−1) under stirring magnetically. After centrifugation, washed thoroughly with water and dried at 60 °C for 10 h, H2PtCl6 aqueous solution was added to a suspension of Er3+:Y3Al5O12/PdS–ZnS dispersed in H2O (10 mL). The suspension was heated to boiling point and kept constant temperature for 15 min. After centrifugation, washed thoroughly with water until Cl− was below detection, and dried at 60 °C for 12 h. The obtained powders were calcined in vacuum at 200 °C for 3.0 h. Finally, the Er3+:Y3Al5O12/Pt–PdS/ZnS photocatalysts were obtained. For the purpose of comparison, Pt–PdS/ZnS were also prepared in the same way.
2.4. Analytical method
The prepared Er3+:Y3Al5O12, Pt–PdS/ZnS and Er3+:Y3Al5O12/Pt–PdS/ZnS were characterized by powder X-ray diffraction (XRD, D-8, Bruker-axs, Germany) using Ni filtered Cu κα radiation in the range of 2θ from 10° to 70°), scanning electron microscopy (SEM, JEOL JSM-5610LV, Hitachi Corporation, Japan), transmission electron microscopy (TEM, JEOL JEM2100, Hitachi Corporation, Japan), energy dispersive X-ray spectroscopy (EDX, JEOL JSM-5610LV, Hitachi Corporation, Japan), X-ray photoelectron spectroscopy (XPS, Escalab 250XI, Thermo, America), UV-vis absorption spectra (UV-2450, Shimadzu, Japan) and PL spectra (RF-5301PC, Shimadzu, Japan).2.5. Visible-light photocatalytic H2 production experimental
The photocatalytic H2 production experiments were performed in a 500 mL Pyrex reactor at ambient temperature and atmospheric pressure. The headspace of the reactor was connected to an inverted burette which is filled with water, allowing the measurement of the evolved hydrogen gas. In a typical photocatalytic experiment, Er3+:Y3Al5O12/Pt–PdS–ZnS powder as photocatalysts was dispersed under constant stirring in an aqueous solution containing 0.20 mol L−1 Na2S and 0.30 mol L−1 Na2SO3. Before irradiation, the system was bubbled with argon for 30 min to remove the dissolved air inside in order to ensure the reaction system was under an anaerobic condition. Then, the suspensions were irradiated for 5.0 h using a 300 W xenon lamp (LX-300, Deruifeng hardware electrical appliance businesses, China) applied as light source, which was positioned on the side of photoreactor. The irradiation wavelength was controlled by a combination of a cold mirror (CM-1) and a water filter (350 < λ < 800 nm). For visible-light irradiation, a cut off filter (L42) was fitted to the aforementioned light source (400 < λ < 800 nm). The formation of hydrogen was confirmed by injecting 0.50 mL of the reactor headspace gas in a gas chromatograph (GC-8A, MS-5A column, TCD, Ar Carrier, Shimadazu, Japan).3. Results and discussions
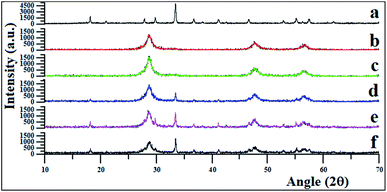
3.1. XRD, SEM, TEM, EDX and XPS of prepared Er3+:Y3Al5O12/Pt–PdS/ZnS
Fig. 1 shows XRD patterns of synthesized Er3+:Y3Al5O12 up-conversion luminescence agent and prepared Pt–PdS/ZnS and Er3+:Y3Al5O12/Pt–PdS/ZnS photocatalysts. The Er3+:Y3Al5O12 (Fig. 1(a)) was identified according to JCPDS #33-0040 file of Y3Al5O12. It demonstrated that we had well synthesized the Er3+:Y3Al5O12, in which the Er3+ ions entered the crystal lattice, taking the place of the partial Y3+ ion. As shown in Fig. 1(b), three pronounced peaks at 28.6° (111), 47.5° (220) and 56.3° (311) in sample can be indexed to the cubic sphalerite ZnS (JCPDS # 05-0566), and no impurity peaks are observed. The broad diffraction peaks of ZnS are due to its small crystallite size. Fig. 1(d–f) shows the characteristic peaks of both cubic sphalerite ZnS and Er3+:Y3Al5O12. From these XRD patterns, we can see the main five peaks at 2θ = 18.12° (211), 33.36° (400), 42.58° (422), 46.62° (521) and 55.12°(532) corresponding to the Er3+:Y3Al5O12, suggesting that the Er3+:Y3Al5O12 part exists in these samples. These patterns of ZnS and Er3+:Y3Al5O12 consist of two sets of diffraction peaks, and the broadening peaks match well with ZnS. And yet the intensity of all diffraction peaks of Er3+:Y3Al5O12 in Er3+:Y3Al5O12/Pt–PdS/ZnS powder weakens obviously as against that of pure Er3+:Y3Al5O12 powder. The characteristic diffraction peaks of Pt and PdS from Fig. 1(c–f) reveal that only 0.30 wt% Pt and 0.13 wt% PdS loaded on the surface of ZnS nanocrystals are not enough to produce the characteristic peaks in XRD patterns.Fig. 2 displays typical SEM image for Er3+:Y3Al5O12 (Fig. 2(a)), Pt–PdS/ZnS (Fig. 2(b)) and Er3+:Y3Al5O12/Pt–PdS/ZnS (Fig. 2(c)). From the image of Er3+:Y3Al5O12 depicted in Fig. 2(a), numerous homogeneous sphere shaped particles can be seen clearly. The image Fig. 2(b) shows monodispersed irregular spheres with an average diameter of ca. 50 nm, confirming the ZnS nanospheres, while the surfaces of the nanospheres are loaded with numerous particles of diameter less than 5 nm. These particles can be verified as PdS and Pt nanoparticles. Both particles are dispersed on the surface of ZnS. As shown in Fig. 2(c), the size of particles is different, among them most of the particles' is about 50 nm. However, there are not some slightly greater particles which can be regarded as the Er3+:Y3Al5O12 (Fig. 2(a). Because the structure of Er3+:Y3Al5O12 is loose, after the treatment of ultrasound grind and boil it can be covered on the surface of ZnS. These findings justify that the Er3+:Y3Al5O12 has been merged with ZnS. In addition, the PdS and Pt have also been stored on them.
Fig. 2(d) shows the TEM of Er3+:Y3Al5O12/Pt–PdS/ZnS photocatalyst. It can be seen that the ZnS displays the slightly regular shape with the average diameter of about 50 nm. Besides, PdS and Pt nanoparticles as cocatalysts are randomly dispersed on the surface of ZnS particles. The typical particle sizes of Pt and PdS are estimated to be ca. 3–5 nm and 10–15 nm, respectively. Particularly, some nanoparticles with a diameter around 15–20 nm are Er3+:Y3Al5O12, which are dispersedly deposited on the surface of ZnS particles.
As shown in Fig. 3, the composition of the as-prepared products is confirmed by energy-dispersive X-ray spectroscopy (EDX) analysis. As displayed in Fig. 3(a), the results of EDX analysis show that the peaks of Er, Y, Al, and O elements are observed without any other peaks and the atomic ratio of Y, Al, and O is close to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, which is in agreement with the stoichiometric ratio of Er3+:Y3Al5O12. The Fig. 3(b) shows that the products predominantly contain Zn and S elements, with a tiny amount of Pt and Pd (Pt and Pd signals arose from depositing small amounts of Pt and PdS on the ZnS). The Fig. 3(c) also indicate that Er, Y, Al, O, Zn, S, Pt and Pd elements are coexisting in the quaternary catalyst close to the stoichiometric ratio further confirming successful fabrication of Er3+:Y3Al5O12/Pt–PdS/ZnS. These results were consistent with the XRD pattern presented above simultaneously.
12, which is in agreement with the stoichiometric ratio of Er3+:Y3Al5O12. The Fig. 3(b) shows that the products predominantly contain Zn and S elements, with a tiny amount of Pt and Pd (Pt and Pd signals arose from depositing small amounts of Pt and PdS on the ZnS). The Fig. 3(c) also indicate that Er, Y, Al, O, Zn, S, Pt and Pd elements are coexisting in the quaternary catalyst close to the stoichiometric ratio further confirming successful fabrication of Er3+:Y3Al5O12/Pt–PdS/ZnS. These results were consistent with the XRD pattern presented above simultaneously.
XPS spectra of Er3+:Y3Al5O12/Pt–PdS/ZnS photocatalyst is shown in Fig. 4. It indicates that this photocatalyst contains Zn, S, Pt, Pd, Er, Y, Al and O elements and the corresponding peaks are plotted carefully. The Fig. 4 shows that the characteristic peaks of Zn (1046 eV 2p1/2 and 1023 eV 2p3/2) and S (163 eV 2p3/2 and 227 eV 2s) can be clearly observed in the wide scan spectrum and as shown in Fig. 4(a and b), the binding energy peaks located at 73 eV, 75 eV, 337 eV and 340 eV well match with the reported values of Pt 4f5/2, Pt 4f7/2, Pd 3d5/2 and Pd 3d3/2 XPS peaks.39,40 These implied that Pt and PdS were successfully decorated on ZnS. Besides these characteristic peaks, the figure also presents other peaks at 173 eV (Er 4d5/2), 294 eV (Y 3p3/2), 147 eV (Y 3d3/2), 118 eV (Al 2s), 91 eV (Al 2p3/2,1/2), 531 eV (O 1s) and 24 eV (O 2s), which closely agree with the composition of Er3+:Y3Al5O12. All peaks of the photocatalyst emerge with a slightly shift due to the efficient charge transfer between the adjacent components during the photocatalytic reaction.
 | ||
Fig. 4 XPS spectra of Er3+:Y3Al5O12/Pt–PdS/ZnS (0.13 wt% PdS and 0.3 wt% Pt contents and 0.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 Er3+:Y3Al5O12 and ZnS mass ratio). 1.00 Er3+:Y3Al5O12 and ZnS mass ratio). | ||
The absorption and PL spectra of up-conversion luminescence agent Er3+:Y3Al5O12 are illustrated in Fig. 5. From the Fig. 5(a), it can be seen that three typical peaks located at 410 nm, 524 nm, 653 nm in absorption spectra. Fig. 5(b–d) shows the PL spectra of Er3+:Y3Al5O12 under the excitation wavelengths of 410 nm, 524 nm and 653 nm, respectively, where the absorption peaks can be obviously found in the absorption spectra. The emission peaks are conspicuous in the ultraviolet-light region (250–400 nm). Therefore, it can be confirmed that the Er3+:Y3Al5O12 as up-conversion luminescence agent can absorb the visible lights and emit the ultraviolet lights. These findings prove that the ZnS combined with Er3+:Y3Al5O12 will be activated effectively by visible light to enhance the effect of photocatalytic H2 production.
 | ||
| Fig. 5 Absorption spectrum (a) and PL spectra (b–d) of Er3+:Y3Al5O12 (heat-treated at 1100 °C for 120 min) at various excitation wavelengths. | ||
3.2. The influence of Er3+:Y3Al5O12 and ZnS mass ratio on visible-light photocatalytic H2 production activity of Er3+:Y3Al5O12/Pt–PdS/ZnS
In preliminary experiments, the study of the influence of the amount of up-conversion luminescence agent (Er3+:Y3Al5O12) in the system was performed in order to optimize the conditions of visible-light photocatalytic H2 production. The results are presented in Fig. 6. It shows that the effects of Er3+:Y3Al5O12 amount on the photocatalytic activity of the Er3+:Y3Al5O12/Pt–PdS/ZnS photocatalysts. The amount of the photocatalytic H2 production continues to enhance with an increase of Er3+:Y3Al5O12 amount (from 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 mass ratio to 0.30
1.00 mass ratio to 0.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 mass ratio) in the Er3+:Y3Al5O12/Pt–PdS/ZnS photocatalysts, and then it decreases with the further increase of Er3+:Y3Al5O12 amount (0.45
1.00 mass ratio) in the Er3+:Y3Al5O12/Pt–PdS/ZnS photocatalysts, and then it decreases with the further increase of Er3+:Y3Al5O12 amount (0.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 mass ratio). All of the Er3+:Y3Al5O12/Pt–PdS/ZnS photocatalysts present higher photocatalytic H2 production rate than that over Pt–PdS/ZnS, revealing that the Er3+:Y3Al5O12 amount affects largely the photocatalytic activity of Pt–PdS/ZnS. The appropriate increase of Er3+:Y3Al5O12 amount can offer much more ultraviolet-light to activate ZnS, which results in the increase of the amount of photocatalytic H2 production. However, the further increase of Er3+:Y3Al5O12 amount relatively leads to the decrease of the amount of photocatalytic H2 production due to the fact that the active surface of ZnS nanospheres decreases.
1.00 mass ratio). All of the Er3+:Y3Al5O12/Pt–PdS/ZnS photocatalysts present higher photocatalytic H2 production rate than that over Pt–PdS/ZnS, revealing that the Er3+:Y3Al5O12 amount affects largely the photocatalytic activity of Pt–PdS/ZnS. The appropriate increase of Er3+:Y3Al5O12 amount can offer much more ultraviolet-light to activate ZnS, which results in the increase of the amount of photocatalytic H2 production. However, the further increase of Er3+:Y3Al5O12 amount relatively leads to the decrease of the amount of photocatalytic H2 production due to the fact that the active surface of ZnS nanospheres decreases.
3.3. The influence of photocatalyst amount on visible-light photocatalytic H2 production of Er3+:Y3Al5O12/Pt–PdS/ZnS and Pt–PdS/ZnS
Fig. 7 shows the influence of the amount of photocatalysts on the rate of visible-light photocatalytic H2 evolution of Er3+:Y3Al5O12/Pt–PdS/ZnS. It can be found that, at beginning, the amounts of H2 evolution increase along with the increase of amount for both Er3+:Y3Al5O12/Pt–PdS/ZnS and Pt–PdS/ZnS photocatalysts up to a certain weight (0.20 g L−1), and then become a sharply decrease with further increase of catalyst amounts. Maybe, it is due to the fact that no more photons could be absorbed to carry out the photocatalytic H2 evolution, because of scattering and reflection of lights caused by overmuch catalyst particles. All in all, the reason is the combination effect of thermodynamic and kinetic factors of the photoelectron generation and transfer in photocatalytic reactions. An optimal amount of photocatalysts is required in order to attain the highest photocatalytic H2 evolution efficiency.3.4. Photocatalytic activity comparison of Er3+:Y3Al5O12/Pt–PdS/ZnS and Pt–PdS/ZnS in visible-light photocatalytic H2 production
Fig. 8 shows the comparison of H2 production amounts catalysed by Er3+:Y3Al5O12/Pt–PdS/ZnS and Pt–PdS/ZnS along with visible-light irradiation time. It can be found that, obviously, the amounts of H2 evolution both significantly increase with the lengthening of visible-light irradiation time for two photocatalysts. However, the amount by using Er3+:Y3Al5O12/Pt–PdS/ZnS is much more than that by using Pt–PdS/ZnS at any visible-light irradiation time. It can be considered that, because of the presence of Er3+:Y3Al5O12 possessing up-conversion luminescence effect from visible-light to ultraviolet-light, it can provide more ultraviolet-light to stimulate ZnS to carry out the photocatalytic H2 production. It reveals that the presence of Er3+:Y3Al5O12 could dramatically improve the visible-light photocatalytic H2 production activity of the Pt–PdS/ZnS catalysts.3.5. The influence of visible-light irradiation intensity on photocatalytic H2 production effect of Er3+:Y3Al5O12/Pt–PdS/ZnS
In this section, the impact of the irradiation intensity on the visible-light photocatalytic H2 production of Er3+:Y3Al5O12/Pt–PdS/ZnS is studied and the corresponding data are presented in Fig. 9. It can be found that the amounts of H2 production both dramatically increase along with the increase of visible-light irradiation intensity for Er3+:Y3Al5O12/Pt–PdS/ZnS and Pt–PdS/ZnS. It indicates that the high-intensity visible-light irradiation is beneficial to the photocatalytic H2 production of Er3+:Y3Al5O12/Pt–PdS/ZnS and Pt–PdS/ZnS. Of course, under the same intensity visible-light irradiation the amounts of H2 production of Er3+:Y3Al5O12/Pt–PdS/ZnS photocatalyst is much more than that of Pt–PdS/ZnS powder. It may be attributed to the fact that the Er3+:Y3Al5O12 offers more ultraviolet-light to ZnS.3.6. Possible visible-light photocatalytic H2 production mechanism and process
Based on the above results, a proposed mechanism for visible-light photocatalytic H2 production from aqueous solution by Er3+:Y3Al5O12/Pt–PdS/ZnS is illustrated in Fig. 10. Firstly, ZnS as a wide band gap photocatalyst plays important role in the photocatalytic H2 production reaction. In addition, two main reasons for the increase in the photocatalytic activity of Er3+:Y3Al5O12/Pt–PdS/ZnS were (1) the presence of up-conversion luminescence agent (Er3+:Y3Al5O12), and (2) the effect of Pt and PdS acting as co-catalyst. The two factors might generate synergistic effect for the enhancement of photocatalytic activity of ZnS. The followings are the detailed interpretation of the main reasons. | ||
| Fig. 10 The schematic diagram of photocatalytic H2 production over quaternary Er3+:Y3Al5O12/Pt–PdS/ZnS photocatalysts under visible-light irradiation. | ||
(1) The up-conversion luminescence agent (Er3+:Y3Al5O12) absorbs the visible-light, and then continuously emits the ultraviolet-light. The Er3+ ion has abundant energy levels in the ultraviolet range, which allows a variety of up-conversion to occur through absorption of low energy photons by an already excited Er3+ ion. Besides, two excited Er3+ ions can exchange energy, such that one of the ions is further excited to an even higher energy state, while the other relaxes to a lower state. That is to say, the up-conversion process can be achieved through the chains of ground state absorption (GSA) and excited state absorption (ESA).41,42 Therefore, under continuous excitation of visible-light, the Er3+:Y3Al5O12 emits the ultraviolet-light, which can effectively excite ZnS to generate electron–hole pairs.
(2) ZnS coloaded with Pt and PdS shows considerably synergistic effect on the photocatalytic H2 production. Upon ultraviolet-light excitation, the photogenerated electron and hole are produced in the conduction band (CB) and valence band (VB) of ZnS, respectively. Pt is effective reduction co-catalysts, while PdS is effective oxidation co-catalyst. The electrons and holes can be separated and transfer efficiently for promoting the photocatalytic activity of ZnS. The holes are consumed by the 0.20 mol L−1 Na2S and 0.30 mol L−1 Na2SO3 as sacrificial agent. And then, the electrons will transfer to the surface of ZnS and reduce protons to H2.
Through these experimental observations, it can be believed that the above two supposed factors are the main reasons that lead to the enhanced activity of the Er3+:Y3Al5O12/Pt–PdS/ZnS photocatalyst.
4. Conclusions
In summary, a quaternary photocatalyst (Er3+:Y3Al5O12/Pt–PdS/ZnS) was successfully prepared by sol–gel and hydrothermal methods. The visible-light photocatalytic H2 production activity of Er3+:Y3Al5O12/Pt–PdS/ZnS from aqueous solution containing 0.20 mol L−1 Na2S and 0.30 mol L−1 Na2SO3 as sacrificial reagents was evaluated under visible-light irradiation. The results indicated that the visible-light photocatalytic H2 production activity of ZnS can be significantly enhanced in the presence of the up-conversion luminescence agent (Er3+:Y3Al5O12). Moreover, the loading of Pt and PdS, as reduction and oxidation co-catalysts, respectively, can help the separation and transfer of photogenerated electrons and holes in ZnS for photocatalytic reactions, significantly promoting the visible-light photocatalytic activity. The visible-light photocatalytic activity of prepared Er3+:Y3Al5O12/Pt–PdS/ZnS is related to the mass ratio of Er3+:Y3Al5O12 and ZnS, catalyst amount and irradiation intensity. Notably, it is the first time to use quaternary composite compound as an effective and stable photocatalyst for H2 production, which provides new insight to enhance the photocatalytic H2 production activity under solar light.Acknowledgements
The authors greatly acknowledge the National Science Foundation of China (21371084), Innovation Team Project of Education Department of Liaoning Province (LT2012001), Public Research Fund Project of Science and Technology Department of Liaoning Province (2012004001), Shenyang Science and Technology Plan Project (F12-277-1-15 and F13-289-1-00) and Science Foundation of Liaoning Provincial Education Department (L2011007) for financial support. The authors also thank our colleagues and other students for their participating in this work.Notes and references
- R. D. Cortright, R. R. Davda and J. A. Dumesic, Nature, 2002, 418, 964–967 CrossRef CAS PubMed.
- Z. Y. Shen, G. Chen, Q. Wang, Y. G. Yu, C. Zhou and Y. Wang, Nanoscale, 2012, 4, 2010–2017 RSC.
- Y. P. Xie, Z. B. Yu, G. Liu, X. L. Ma and H. M. Cheng, Energy Environ. Sci., 2014, 7, 1895–1901 CAS.
- N. Z. Bao, L. M. Shen, T. Takata and K. Domen, Chem. Mater., 2008, 20, 110–117 CrossRef CAS.
- T. P. Xie, C. L. Liu, L. J. Xu, J. Yang and W. Zhou, J. Phys. Chem. C, 2013, 117, 24601–24610 CAS.
- Y. T. Lu, D. D. Wang, P. Yang, Y. K. Du and C. Lu, Catal. Sci. Technol., 2014, 4, 2650–2657 CAS.
- X. B. Chen, S. H. Shen, L. J. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503–6570 CrossRef CAS PubMed.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- K. Zhang and L. J. Guo, Catal. Sci. Technol., 2013, 3, 1672–1690 CAS.
- F. Z. Jia, Z. P. Yao and Z. H. Jiang, Int. J. Hydrogen Energy, 2012, 37, 3048–3055 CrossRef CAS.
- C. H. Deng, X. Q. Ge, H. M. Hu, L. Yao, C. L Han and D. F. Zhao, CrystEngComm, 2014, 16, 2738–2745 RSC.
- M. Shen, Z. P. Yan, L. Yang, P. W. Du, J. Y. Zhang and B. Xiang, Chem. Commun., 2014, 50, 15447–15449 RSC.
- S. Martha, D. K. Padhi and K. Parida, ChemSusChem, 2014, 7, 585–597 CrossRef CAS PubMed.
- Y. S. Zhou, G. Chen, Y. G. Yu, Y. J. Feng, Y. Zheng, F. He and Z. H. Han, Phys. Chem. Chem. Phys., 2015, 17, 1870–1876 RSC.
- A. D. Mani, P. Ghosal and C. Subrahmanyam, RSC Adv., 2014, 4, 23292–23298 RSC.
- N. X. Li, L. Z. Zhang, J. C. Zhou, D. W. Jing and Y. M. Sun, Dalton Trans., 2014, 43, 11533–11541 RSC.
- S. J. Hu, L. C. Jia, B. Chi, J. Pu and L. Jian, J. Power Sources, 2014, 266, 304–312 CrossRef CAS.
- J. L. Yuan, J. Q. Wen, Q. Z. Gao, S. C. Chen, J. M. Li, X. Li and Y. P. Fang, Dalton Trans., 2015, 44, 1680–1689 RSC.
- J. Zhang, S. W. Liu, J. G. Yu and M. Jaroniec, J. Mater. Chem., 2011, 21, 14655–14662 RSC.
- J. R. Ran, J. Zhang, J. G. Yu, M. Jaroniec and S. Z. Qiao, Chem. Soc. Rev., 2014, 43, 7787–7812 RSC.
- S. Liu, X. T. Wang, K. Wang, R. Lv and Y. L. Xu, Appl. Surf. Sci., 2013, 283, 732–739 CrossRef CAS.
- Z. Wang, S. W. Cao, S. C. J. Loo and C. Xue, CrystEngComm, 2013, 15, 5688–5693 RSC.
- Z. L. Wang, J. H. Hao and H. L. W. Chan, J. Mater. Chem., 2010, 20, 3178–3185 RSC.
- F. M. Cheng, K. N. Sun, Y. Zhao, Y. J. Liang, Q. Xin and X. L. Sun, Ceram. Int., 2014, 40, 11329–11334 CrossRef CAS.
- J. Zhou, W. X. Zhang, J. Li, B. X. Jiang, W. B. Liu and Y. B. Pan, Ceram. Int., 2010, 36, 193–197 CrossRef CAS.
- C. Kong, S. X. Min and G. X. Lu, Chem. Commun., 2014, 50, 9281–9283 RSC.
- J. Di, J. X. Xia, Y. P. Ge, L. Xu, H. Xu, J. Chen, M. Q. He and H. M. Li, Dalton Trans., 2014, 43, 15429–15438 RSC.
- M. Barawi, I. J. Ferrer, J. R. Ares and C. Sánchez, ACS Appl. Mater. Interfaces, 2014, 6, 20544–20549 CAS.
- J. H. Yang, H. J. Yan, X. L. Wang, F. Y. Wen, Z. J. Wang, D. Y. Fan, J. Y. Shi and C. Li, J. Catal., 2012, 290, 151–157 CrossRef CAS.
- R. G. Li, H. X. Han, F. X. Zhang, D. G. Wang and C. Li, Energy Environ. Sci., 2014, 7, 1369–1376 CAS.
- J. C. Boyer, F. Vetrone, L. A. Cuccia and J. A. Capobianco, J. Am. Chem. Soc., 2006, 128, 7444–7445 CrossRef CAS PubMed.
- G. J. Feng, S. W. Liu, Z. L. Xiu, Y. Zhang, J. X. Yu, Y. G. Chen, P. Wang and X. J. Yu, J. Phys. Chem. C, 2008, 112, 13692–13699 CAS.
- B. L. Zhu, B. Z. Lin, Y. Zhou, P. Sun, Q. R. Yao, Y. L. Chen and B. F. Gao, J. Mater. Chem. A, 2014, 2, 3819–3827 CAS.
- J. Wang, Y. F. Lim and G. W. Ho, Nanoscale, 2014, 6, 9673–9968 RSC.
- J. Y. Zhang, Y. H. Wang, J. Zhang, Z. Lin, F. Huang and J. G. Yu, ACS Appl. Mater. Interfaces, 2013, 5, 1031–1037 CAS.
- H. J. Yan, J. H. Yang, G. J. Ma, G. P. Wu, X. Zong, Z. B. Lei, J. Y. Shi and C. Li, J. Catal., 2009, 266, 165–168 CrossRef CAS.
- Y. H. Pai, C. T. Tsai and S. Y. Fang, J. Power Sources, 2013, 223, 107–113 CrossRef CAS.
- J. L. Meng, Z. M. Yu, Y. Li and Y. D. Li, Catal. Today, 2014, 225, 136–141 CrossRef CAS.
- Q. Gu, J. L. Long, H. Q. Zhuang, C. Q. Zhang, Y. Q. Zhou and X. X. Wang, Phys. Chem. Chem. Phys., 2014, 16, 12521–12534 RSC.
- J. Batista, A. Pintar, D. Mandrino, M. Jenko and V. Martin, Appl. Catal., A, 2001, 206, 113–124 CrossRef CAS.
- N. N. Zu, H. G. Yang and Z. W. Dai, Phys. B, 2008, 403, 174–177 CrossRef CAS.
- Z. J. Zhang, W. Z. Wang, J. Xu, M. Shang, J. Ren and S. M. Sun, Catal. Commun., 2011, 13, 31–34 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |