Characterization and electrochemical properties of a nickel film/carbon paper electrode prepared by a filtered cathodic vacuum arc technique
Yingyi
Fu
a,
Wen
Su
a,
Tong
Wang
a and
Jingbo
Hu
*ab
aCollege of Chemistry, Beijing Normal University, Beijing 100875, PR China. E-mail: hujingbo@bnu.edu.cn; Fax: +86 10 58802075; Tel: +86 10 62209398
bKey Laboratory of Beam Technology and Material Modification of Ministry of Education, Beijing Normal University, Beijing 100875, PR China
First published on 16th June 2015
Abstract
A nickel film was prepared through plasma deposition of a metal onto a carbon paper (CP) substrate with the filtered cathodic vacuum arc technique. Nickel metal plasma was generated at a current of 90 A and deposited on the CP substrate for 3 seconds, forming the nickel film modified electrode. The morphology image of the nickel film on the substrate surface was characterized by scanning electron microscopy (SEM). The existence of the nickel film was verified by energy dispersive spectroscopy (EDS) and X-ray photoelectron spectroscopy (XPS). The results of the water contact angle measurement (WCA) showed that the existence of the nickel film improved the hydrophilicity of the CP. Cyclic voltammetry (CV) was carried out to investigate the electrochemical properties of the Ni/CP electrode. The nickel film provided a good electron conduction pathway and it improved the electron transfer ability of the substrate. It was found that the Ni/CP electrode exhibited good electrocatalytic oxidation behaviour towards glucose. Amperometric responses showed a good linear relationship with glucose concentration in the range from 2 μM to 500 μM with a detection limit of 0.6 μM. Thus this material is expected to have wide potential applications in glucose biosensors.
1. Introduction
Ni-based materials have drawn great attention in battery cathodes, catalysts and biosensors due to their low toxicity, good electrochemical stability and catalytic properties.1–4 Ni materials have a unique electrocatalytic effect which arises from the unpaired d electrons and vacant d-orbitals. It is also well known that nickel and nickel hydroxide exhibit electrochemical activity in alkaline medium.5–7 There are several methods to fabricate Ni material, such as electrochemical deposition,8 chemical reduction,9 seed-mediated growth10 and electrospinning.11 However, the need of chemical reagents and the complex process of these methods are unfavourable for the electrode preparation. In the present paper, we fabricate nickel thin film on the carbon paper (CP) surface with a new method, which is filtered cathodic vacuum arc technique.The filtered cathodic vacuum arc is an emerging technology which has been used for various kinds of materials, such as metals, metal oxides and metal nitrides, doped and undoped semiconductors.12–18 The vacuum cathodic arc has been employed extensively as a method of fabricating thin film with origin that can be traced back 1892 with a patent of Edison.19,20 This technique utilizes the cathode vacuum arc discharge and produces high density metal plasma. However, there are some macroparticles in the produced metal plasma. In order to obtain high quality thin film, these macroparticles must be removed. There are a few methods to remove the macroparticles, such as using mechanical blades filter, pulsed technique and magnetic filter.21–23 Magnetic filter method has been used extensively and we adopted it to achieve the goal. Metal plasma will move along the direction of magnetic field and reach the vacuum chamber, however the macroparticles won't.24,25 By means of magnetic filter technique, we can obtain homogeneous metal plasma. Finally, the metal plasma will be deposited on the CP in vacuum chamber in a very short time. Compared this technique with other modification methods which mentioned above, such as electrochemical deposition and chemical reduction, this technique can modify the wanted films on the substrate materials directly without any chemical reagent or complex process. The modification process is facile and fast. Besides, the magnetic filter technique provides modified electrode with long-term stability and the thickness of films can be controlled by operating conditions.23,26–30
CP is a kind of carbon fiber material, which has good compatibility, high specific surface area, excellent electrical conductivity, low resistivity and stable chemical properties. Besides, low cost carbon paper, unlike other substrate materials, can be cut out in any size at any time.31–34 These properties of CP make it a suitable substrate material.
In the present paper, we selected CP as a substrate and deposited nickel thin film on it by filtered cathodic vacuum arc technique. The Ni/CP material was characterized by scanning electron microscope (SEM), energy dispersive spectroscopy (EDS), X-ray photoelectron spectra (XPS) and water contact angle measurement (WCA). As an electrode material, the electrochemical behaviour of Ni/CP was revealed by cyclic voltammetry (CV) and chronoamperometry. The results showed that the modified electrode Ni/CP exhibited ideal electrochemical and electrocatalytic properties.
2. Experimental
2.1. Apparatus
Filtered cathodic vacuum arc deposition was carried out with the Beijing Normal University (BNU) FAD-MEVVA. X-ray photoelectron spectroscopy (XPS) data was taken on an AXIS Ultra spectrometer (Shimadzu, Japan). The scanning electron microscope (SEM) and energy dispersive spectroscopy (EDS) results were acquired with Hitachi X650 (Hitachi X650, Japan). The water contact angle measurement (WCA) was performed with the OCA15EC contact angle goniometer (Dataphysics Instruments GmbH, Germany). All of the electrochemical measurements were performed with a CHI660D electrochemical workstation (CH Instruments, USA) at room temperature. A traditional three-electrode system was applied with a bare or modified carbon paper electrode (A = 30 mm2) as the working electrode, an Ag/AgCl electrode (saturated KCl) as the reference electrode and a platinum wire electrode as the counter electrode.2.2. Reagents
CP was purchased from Shanghai Hephas Energy Company Limited. All of the chemical reagents were of analytical grade and solutions were dissolved with triple distilled water. All of the measurements were carried out at room temperature.2.3. Fabrication of nickel film on carbon paper
Filtered cathodic vacuum arc deposition was carried out using BNU FAD-MEVVA. High density nickel plasma was produced using cathodic vacuum arc discharge technique at 90 A. Macroparticles in metal plasma can be removed through the magnetic filter. After that, nickel plasma was deposited on the CP surface at 0 V. The deposition process continued for 3 seconds, forming the Ni/CP electrode. Before being used, the modified electrode was washed with distilled water and ethanol for several times.3. Results and discussion
3.1. Morphological characterization
The morphologies of the Ni/CP electrode were characterized by scanning electron microscopy (SEM). As can be seen in Fig. 1A and B, we can obtain the rough morphology of CP that many interlaced tubular carbon fibres dispersed randomly. In the cross-sectional views of carbon paper, we can see that the carbon fibres stacked optionally and the interval between each other is different. In high magnification of SEM, we can observe that the surface is not smooth and there are many grooves on the surface of CP (Fig. 1C). After deposition, no obvious morphology changes are observed and the tubular carbon fibres still exist (Fig. 1D). This phenomenon might be due to the characteristic of the technique which modifies the electrode without damage. In Fig. 1D, no macroparticles on the carbon paper are observed after deposition, which demonstrates that the magnetic filter method works effectively. The EDS spectrums (Fig. 1E and F) indicate the presence of Ni on the surface of CP after modification. These results above demonstrate that the Ni film was modified on the surface of CP successfully.3.2. XPS measurement of Ni/CP electrode
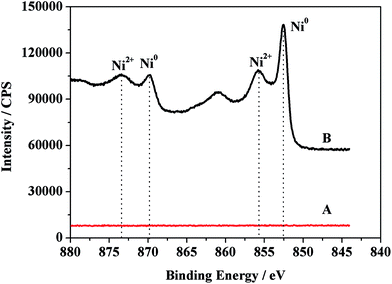
The chemical composition of the Ni/CP electrode was studied by XPS further (Fig. 2). As shown in Fig. 2, there are no nickel peaks on the CP electrode (curve A). However, two typical Ni 2p peaks are observed at about 852.5 eV (2p3/2) and 869.8 eV (2p1/2) (curve B), with a spin–orbit splitting of 17.3 eV. The data demonstrate that the nickel thin film immobilized on the electrode surface is in the zero valent metallic state (Ni0). The satellite peak at 861.1 eV corresponds to Ni (2p3/2). In addition, Ni 2p3/2 peak at about 855.8 eV and Ni 2p1/2 at about 873.5 eV indicate the existence of Ni2+ on the electrode.35–39 From the results above, it can be concluded that Ni0 and Ni2+ both exist on the nickel film surface.The XPS spectra can also check the chemical states of oxygen element in the CP and Ni/CP electrode. As it can be seen from the O 1s spectra of CP electrode (Fig. 3A), the peak at 531.8 eV corresponds to C–O, the O concentration on the surface of CP is approximately 5.3%. After nickel film was modified on CP, the peak O 1s states can be divided into three components (Fig. 3B). The peaks at 532.8 eV, 531.8 eV and 529.3 eV are indications of the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O and metal oxide, respectively.35,40,41 The concentration of O increases to 31.7%, which might originate from the formation of metal oxide. These results are consistent with the Fig. 2, metal nickel exists in the form of zero valent and metal oxide state on the CP.
O, C–O and metal oxide, respectively.35,40,41 The concentration of O increases to 31.7%, which might originate from the formation of metal oxide. These results are consistent with the Fig. 2, metal nickel exists in the form of zero valent and metal oxide state on the CP.
3.3. WCA measurement of Ni/CP electrode
WCA measurements were applied to evaluate the surface hydrophilicity of both the original CP electrode and the Ni/CP electrode (Fig. 4). The measurements were carried out with 2 μL water droplet placed on the surface of the electrode. The contact angles of the CP and Ni/CP electrodes were 130.7° and 118.1°, respectively. The smaller contact angle value of Ni/CP electrode compared to that of the CP electrode illustrates that the modification of the nickel film improves the hydrophilicity of the substrate. The increase in hydrophilicity of Ni/CP electrode benefits to the reaction of electroactive substance on the electrode surface.3.4. Electrochemical characterization of Ni/CP electrode
CV was employed to investigate the electrochemical behaviour of the CP and Ni/CP electrode with the redox system serving as the probe such as [Fe(CN)6]3−/[Fe(CN)6]4−. Fig. 5 showed CVs for an aqueous solution of 5 mM [Fe(CN)6]3−/[Fe(CN)6]4− in 0.1 M KCl at a scan rate of 100 mV s−1. KCl acted as supporting electrolyte in the solution, a mass of anions (Cl−) and cations (K+) surrounded around the anode and the cathode, therefore the electrostatic attraction between electrode and measured ions was reduced and the migration current generated by electrostatic attraction was eliminated. Through added KCl, the migration current was eliminated and the electrode process was control by diffusion completely. These made the experiment result more accurate. CV of the CP electrode exhibits a pair of well-defined oxidation and reduction peak with a peak-to-peak separation (ΔEp) of 323 mV (curve A). After deposition of nickel film on the CP substrate, a pair of characteristic oxidation and reduction peaks was obtained. The anodic and cathodic peak potentials move negatively and positively, with a ΔEp of 270 mV (curve B). Besides, the oxidation and reduction peak currents are higher than those of the CP electrode. Compared to the CP electrode, the decrease of the ΔEp of Ni/CP electrode indicates that the property of Ni/CP is better than CP electrode. These results demonstrate that the modification of nickel film can accelerate the electron transfer rate between the electrode surface and [Fe(CN)6]3−/[Fe(CN)6]4− in aqueous solution and exhibit good electrical properties. So we believe that the nickel film has been successfully deposited on the CP surface and provide good electron conduction pathway which is suitable for electrochemical sensor. | ||
| Fig. 5 Cyclic voltammograms of CP electrode (A) and Ni/CP electrode (B) in 5 mM [Fe(CN)6]3−/4− solution at scan rate of 100 mV s−1. | ||
3.5. The electrocatalytic oxidation of glucose
Nickel material modified electrode exhibits favorable electrocatalytic effects towards some biomolecules.42–44 To characterize the electrocatalytic ability of Ni/CP electrode, the electrooxidation behaviour towards glucose in alkaline aqueous solution was investigated. CVs of the Ni/CP electrode in 0.1 M NaOH aqueous solution in the absence (A) and presence (B) of 1 mM glucose are shown in Fig. 6. Curve A shows a pair of redox peaks of Ni2+/Ni3+ at 0.64 V and 0.50 V in alkaline medium. Upon the addition of 1 mM glucose, the anodic peak current increases and the cathodic peak current decreases (curve B), illustrating that Ni/CP electrode exhibits excellent electrocatalytic ability towards the oxidation of glucose. The increase of oxidation current is ascribed to the production of Ni(OH)2 from the reaction between NiOOH and glucose. The produced Ni(OH)2 is further oxidized to NiOOH at the electrode surface. In addition, the anodic peak potential moves from 0.64 V to 0.67 V and the reason of that may be due to the diffusion limitation of glucose at the electrode surface. As indicated in literatures,45–47 the glucose can be oxidized to gluconolactone by NiOOH, which produced by the oxidation of Ni(OH)2. The mechanism of the glucose oxidation process can be illustrated as follows:| Ni(OH)2 + OH− → NiOOH + H2O + e− |
| NiOOH + glucose → Ni(OH)2 + gluconolactone |
 | ||
| Fig. 6 CVs of the Ni/CP electrode without (A) and with (B) 1 mM glucose in 0.1 M NaOH. Scan rate: 100 mV s−1. | ||
The effect of scan rate on oxidation peak current and reduction peak current with 1 mM glucose has been investigated in the range of 20–300 mV s−1 (Fig. 7). Both the anodic and the cathodic peak current are linearly proportional to the square root of the scan rate, indicating a diffusion-controlled process. The value of electron transfer coefficient (α) can be obtained from the equation of Epa = 0.11862![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v + 0.43502. According to Laviron,48 the slope of the plot of Epaversus the logarithm of scan rate is 2.3RT/(1 − α)nF and the value of α was calculated to be 0.5.
v + 0.43502. According to Laviron,48 the slope of the plot of Epaversus the logarithm of scan rate is 2.3RT/(1 − α)nF and the value of α was calculated to be 0.5.
Fig. 8 presents a typical amperometric response of Ni/CP upon the successive addition of a certain concentration of glucose at the potential of 0.67 V. The amperometric current response was increased linearly with the increasing glucose concentration in the range of 2 to 500 μM with a correlation coefficient of 0.9916. The detection limit is found to be 0.6 μM. The linear range and the detection limit of Ni/CP are compared with those of other modified electrodes in Table 1. The good catalytic effect and the simple preparation process make Ni/CP as a potential material in glucose detection.
4. Conclusions
Carbon paper substrate was deposited with nickel film at 90 A in 3 seconds by the technique of filtered cathodic vacuum arc, forming the Ni/CP electrode. The obtained Ni film was characterized by SEM, EDS, XPS, WCA and CV. The results of them confirmed that the nickel film was deposited on the CP surface successfully and it improved the hydrophilicity and electron transfer ability of the substrate. The Ni/CP electrode exhibited good catalytic ability towards the oxidation of glucose in alkaline solution with wide linear range and low detection limit. Thus, the Ni/CP electrode is a promising material in the development of non-enzymatic glucose sensor.Acknowledgements
This research was supported by the Fundamental Research Funds for the Central Universities (Grant no. 2012YBXS09) and the Key Laboratory of Beam Technology and Material Modification of Ministry of Education, China.References
- C. Li, Y. Liu, L. Li, Z. Du, S. Xu, M. Zhang, X. Yin and T. Wang, Talanta, 2008, 77, 455–459 CrossRef CAS PubMed.
- Y. Liu, H. Teng, H. Hou and T. You, Biosens. Bioelectron., 2009, 24, 3329–3334 CrossRef CAS PubMed.
- C. L. Carnes and K. J. Klabunde, J. Mol. Catal. A: Chem., 2003, 194, 227–236 CrossRef CAS.
- V. Biju and M. Abdul Khadar, J. Mater. Sci. Eng. A, 2001, 304–306, 814–817 CrossRef.
- K. E. Toghill, L. Xiao, M. A. Phillips and R. G. Compton, Sens. Actuators, B, 2010, 147, 642–652 CrossRef CAS.
- Z. Chen, J. Nai, H. Ma and Z. Li, Electrochim. Acta, 2014, 116, 258–262 CrossRef CAS.
- S. M. El-Refaei, M. Awad, B. El-Anadouli and M. Saleh, Electrochim. Acta, 2013, 92, 460–467 CrossRef CAS.
- C.-Y. Tai, J.-L. Chang and J.-M. Zen, Chem. Commun., 2009, 6083–6085 RSC.
- A. Maringa, T. Mugadza, E. Antunes and T. Nyokong, J. Electroanal. Chem., 2013, 700, 86–92 CrossRef CAS.
- G. Wang, X. Lu, T. Zhai, Y. Ling, H. Wang, Y. Tong and Y. Li, Nanoscale, 2012, 4, 3123–3127 RSC.
- F. Cao, S. Guo, H. Ma, D. Shan, S. Yang and J. Gong, Biosens. Bioelectron., 2011, 26, 2756–2760 CrossRef CAS PubMed.
- O. S. Panwar, M. A. Khan, B. Bhattacharjee, A. K. Pal, B. S. Satyanarayana, P. N. Dixit, R. Bhattacharyya and M. Y. Khan, Thin Solid Films, 2006, 515, 1597–1606 CrossRef CAS.
- D. R. Martins, M. C. Salvadori, P. Verdonck and I. G. Brown, Appl. Phys. Lett., 2002, 81, 1969–1971 CrossRef CAS.
- O. S. Panwar, M. A. Khan, S. Kumar, A. Basu, B. R. Mehta, S. Kumar and I. Rawal, Surf. Coat. Technol., 2010, 205, 2126–2133 CrossRef CAS.
- O. S. Panwar, M. A. Khan, M. Kumar, S. M. Shivaprasad, B. S. Satyanarayana, P. N. Dixit and R. Bhattacharyya, Jpn. J. Appl. Phys., 2009, 48, 065501 CrossRef.
- A. Kesarwani, O. Panwar, R. Tripathi, M. Dalai and S. Chockalingam, Mater. Sci. Semicond. Process., 2015, 31, 1–9 CrossRef CAS.
- R. K. Tripathi, O. S. Panwar, A. K. Kesarwani, I. Rawal, B. P. Singh, M. K. Dalai and S. Chockalingam, RSC Adv., 2014, 4, 54388–54397 RSC.
- J. D. Beard, S. Aleksandrov, C. H. Walker, D. Wolverson, J. M. Mitchels and S. N. Gordeev, RSC Adv., 2014, 4, 26635–26644 RSC.
- T. A. Edison, Journal, 1892.
- J. M. Schneider, S. Rohde, W. D. Sproul and A. Matthews, J. Phys. D: Appl. Phys., 2000, 33, R173 CrossRef CAS.
- O. S. Panwar, S. K. Gupta, M. A. Khan, B. S. Satyanarayana and R. Bhattacharyya, Diamond Relat. Mater., 2004, 13, 513–520 CrossRef CAS.
- O. S. Panwar, B. Deb, B. S. Satyanarayana, M. A. Khan, R. Bhattacharyya and A. K. Pal, Thin Solid Films, 2005, 472, 180–188 CrossRef CAS.
- D. M. Sanders and A. Anders, Surf. Coat. Technol., 2000, 133–134, 78–90 CrossRef CAS.
- V. Belous, V. Vasyliev, V. Goltvyanytsya, S. Goltvyanytsya, A. Luchaninov, E. Reshetnyak, V. Strel'nitskij, G. Tolmacheva and O. Danylina, Surf. Coat. Technol., 2011, 206, 1720–1726 CrossRef CAS.
- S. Anders, A. Anders, M. R. Dickinson, R. A. MacGill and I. G. Brown, IEEE Trans. Plasma Sci., 1997, 25, 670–674 CrossRef.
- R. L. Boxman, V. Zhitomirsky, B. Alterkop, E. Gidalevich, I. Beilis, M. Keidar and S. Goldsmith, Surf. Coat. Technol., 1996, 86–87, 243–253 CrossRef CAS.
- A. Wei, D. Chen, N. Ke, S. Peng and S. P. Wong, Thin Solid Films, 1998, 323, 217–221 CrossRef CAS.
- A. Anders, Surf. Coat. Technol., 1999, 120–121, 319–330 CrossRef CAS.
- A. Bendavid and P. Martin, J. Aust. Ceram. Soc., 2014, 50, 86–101 CAS.
- R. Mendelsberg, S. Lim, Y. K. Zhu, J. Wallig, D. Milliron and A. Anders, J. Phys. D: Appl. Phys., 2011, 44, 232003 CrossRef.
- Z. Tai, X. Yan, J. Lang and Q. Xue, J. Power Sources, 2012, 199, 373–378 CrossRef CAS.
- M. Sun, F. Zhang, Z.-H. Tong, G.-P. Sheng, Y.-Z. Chen, Y. Zhao, Y.-P. Chen, S.-Y. Zhou, G. Liu, Y.-C. Tian and H.-Q. Yu, Biosens. Bioelectron., 2010, 26, 338–343 CrossRef CAS PubMed.
- L. Yang, S. Cheng, Y. Ding, X. Zhu, Z. L. Wang and M. Liu, Nano Lett., 2011, 12, 321–325 CrossRef PubMed.
- S. Kim, Y.-S. Kuk, Y. S. Chung, F.-L. Jin and S.-J. Park, J. Ind. Eng. Chem., 2014, 20, 3440–3445 CrossRef CAS.
- T.-R. Ling, C.-S. Li, J.-J. Jow and J.-F. Lee, Electrochim. Acta, 2011, 56, 1043–1050 CrossRef CAS.
- H. B. Li, P. Liu, Y. Liang, J. Xiao and G. W. Yang, CrystEngComm, 2013, 15, 4054–4057 RSC.
- X. Wu, W. Xing, L. Zhang, S. Zhuo, J. Zhou, G. Wang and S. Qiao, Powder Technol., 2012, 224, 162–167 CrossRef CAS.
- L. Ji, Z. Lin, M. Alcoutlabi, O. Toprakci, Y. Yao, G. Xu, S. Li and X. Zhang, RSC Adv., 2012, 2, 192–198 RSC.
- R. Suresh Babu, P. Prabhu and S. Sriman Narayanan, Talanta, 2013, 110, 135–143 CrossRef CAS PubMed.
- S. Chen, J. Duan, J. Ran, M. Jaroniec and S. Z. Qiao, Energy Environ. Sci., 2013, 6, 3693–3699 CAS.
- Q. Li, C.-L. Liang, X.-F. Lu, Y.-X. Tong and G.-R. Li, J. Mater. Chem. A, 2015, 3, 6432–6439 CAS.
- W. Dai, M. Li, H. Li and B. Yang, Sens. Actuators, B, 2014, 201, 31–36 CrossRef CAS.
- R. Ojani, J.-B. Raoof and S. Zamani, Bioelectrochemistry, 2012, 85, 44–49 CrossRef CAS PubMed.
- Y. Zhang, F. Xu, Y. Sun, Y. Shi, Z. Wen and Z. Li, J. Mater. Chem., 2011, 21, 16949–16954 RSC.
- Y. Mu, D. Jia, Y. He, Y. Miao and H.-L. Wu, Biosens. Bioelectron., 2011, 26, 2948–2952 CrossRef CAS PubMed.
- A. Sun, J. Zheng and Q. Sheng, Electrochim. Acta, 2012, 65, 64–69 CrossRef CAS.
- H. Nie, Z. Yao, X. Zhou, Z. Yang and S. Huang, Biosens. Bioelectron., 2011, 30, 28–34 CrossRef CAS PubMed.
- E. Laviron, J. Electroanal. Chem., 1979, 101, 19–28 CrossRef CAS.
- H. Tian, M. Jia, M. Zhang and J. Hu, Electrochim. Acta, 2013, 96, 285–290 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |