A green, simple and cost-effective approach to synthesize high quality graphene by electrochemical exfoliation via process optimization
Chung-Hsien
Chuang
a,
Ching-Yuan
Su
bg,
Kuei-Ting
Hsu
c,
Chia-Hsuan
Chen
b,
Chia-Hung
Huang
de,
Chi-Wen
Chu
df and
Wei-Ren
Liu
*a
aDepartment of Chemical Engineering, Chung Yuan Christian University, Taiwan. E-mail: WRLiu1203@gmail.com
bDepartment of Mechanical Engineering, National Central University, Taiwan
cDepartment of Chemical Engineering, Army Academic R.O.C, Taiwan
dMetal Industries Research and Development Centre, Kaohsiung, Taiwan
eDepartment of Materials Science and Engineering, National Cheng Kung University, Tainan, Taiwan
fDepartment of Green Energy, National University of Tainan, Tainan, Taiwan
gGraduate Institute of Energy Engineering, National Central University, Taiwan
First published on 10th June 2015
Abstract
This research focuses on manufacturing graphene nanosheets (GNSs) using an electrochemical exfoliation method. Through tuning the synthetic parameters, such as the composition of electrolytes, concentration of the electrolyte, KOH/H2SO4 ratio, and applied voltage and current density, the relationships between the material’s characteristics and synthetic parameters were studied. The physical and chemical properties of as-synthesized GNSs were systematically studied using scanning electron microscopy (SEM), high resolution transmission electron microscopy (HR-TEM), X-ray diffraction (XRD), atomic force microscopy (AFM), Raman spectroscopy (Raman), Fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS). Among the synthetic parameters, current density plays a very important role in this exfoliation process. Under the optimal current density of 0.11 A cm−2, we successfully synthesized high quality GNSs with a D/G ratio of 0.061, which is far superior to the reported values of other groups. The results indicate that a green, simple and cost-effective exfoliation process has been successfully developed in this study.
1. Introduction
Graphene, a novel material with just a single layer of carbon atoms in a two-dimensional lattice, was discovered in 2004 by A. K. Geim and K. S. Novoselov through a mechanical method,1 and has attracted tremendous attention for a wide variety of applications. This material possesses unique physical properties, such as intrinsic carrier mobility (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s−1), excellent mechanical strength (∼1100 GPa), superior thermal conductivity (∼5000 W m−1 K−1), high electrical conductivity (10−6 Ω cm) and almost complete transparency in visible light (97.7%).1–4 All of the properties mentioned above make graphene one of the most promising materials for applications in electronics, photonics, composite materials and several other fields.
000 cm2 V−1 s−1), excellent mechanical strength (∼1100 GPa), superior thermal conductivity (∼5000 W m−1 K−1), high electrical conductivity (10−6 Ω cm) and almost complete transparency in visible light (97.7%).1–4 All of the properties mentioned above make graphene one of the most promising materials for applications in electronics, photonics, composite materials and several other fields.
The synthesis of graphene by several routes has been investigated. (1) Mechanical exfoliation results in quite high quality graphene, but it is impossible to scale up its production.1,5 (2) Chemical vapor deposition using copper or nickel as catalysts can grow large-area and high-quality graphene.6–10 However, this technique needs to be performed under strict conditions, which may restrict the material’s production at a reasonable price. (3) Chemical exfoliation based on Hummers’ method can produce graphene derivatives in large quantities.11–14 The oxidation of graphite flakes into graphene oxide (GO) and then reduction by chemical or thermal treatment has become a common process due to its fairly low cost compared to other methods. However, lots of strong chemicals, such as sulfuric acid, hydrochloric acid or potassium permanganate, are applied in this process. This is environmentally unfriendly and hazardous due to possibility of explosion.15 Additionally, the high level of defects and oxygen functional groups present in reduced GO (rGO) makes its physical properties scarcely comparable to graphene. In other words, the shorting of this process is very dangerous and as-synthesized rGO powder is full of structural defects. Herein, we demonstrate a one-step method to obtain high-quality graphene nanosheets through electrochemical exfoliation, which is safer, more time-saving and more environmentally friendly than chemical exfoliation. Moreover, it was reported that electrochemically exfoliated graphene (EC-graphene) is of higher quality.16
In this research, high quality graphene nanosheets with low levels of contamination were manufactured using an electrochemical exfoliation method. High purity graphite plates were subjected to DC to exfoliate the electrodes, then the exfoliated-graphite was shattered with sonication. The findings were analysed and discussed, to provide a better understanding of the synthesis of few-layer graphene with a low level of defects and large lateral size .
2. Experimental section
2.1. Sample preparation
In the past, expandable graphite was prepared by the chemical intercalation of sulfuric acid followed immediately by exposure to a high temperature, where the graphite was expanded by gaseous species released from the intercalant.17,18 For this reason, we considered that adopting sulfuric acid as an electrolyte is a good start. However, some reports indicate that only using sulfuric acid to exfoliate graphite into graphene will generally produce a high level of defects because of intense oxidation. To improve this phenomenon, potassium hydroxide has been added to lower the acidity of the electrolyte solution.9 Other work demonstrates that merely employing potassium hydroxide as an electrolyte will result in graphene of excellent quality.16In this experiment, we combined both compounds mentioned above (sulfuric acid and potassium hydroxide) as electrolytes, and then focused on the concentration of the solution, applied voltage and electrode distance. First, we found out the optimal parameters for the best graphene production, and then carried out further analysis. Table 1 presents the experimental design of the process for the electrochemical exfoliation of graphite in our study. The parameters we chose included voltage, composition of the electrolyte, and the distance between two graphite electrodes. First, electrolytes with different weight proportions of KOH and H2SO4 were prepared, and two graphite plates were set up in parallel, as shown in Fig. 1(a). Then, these electrodes were connected to a DC power supply with wires and submerged in the solution, which was continuously stirred with a magnet to reduce the effect of the concentration polarization phenomenon. A cooling system was applied on the outer side of the electro-bath to avoid raising the temperature. After beginning the electrolysis process, silver-like substances gradually appeared and reunited on the surface of the electrolyte solution, as shown in Fig. 1(b). The silver-like substances were collected with a scoop and the electrolyte was removed through considerable water washing, until the filtrate was around neutral. As a result, exfoliated graphene was obtained. Finally, we treated the samples with sonication to turn exfoliated graphene completely into EC-graphene. Therefore, highly dispersed graphene solutions that exhibited the Tyndall effect were obtained, as shown in Fig. 1(c). After the crossover parameters study, represented by Table 1, we found that when the electrolyte solution had a weight percentage of 30% KOH(aq.) and 13.5% H2SO4(aq.), the distance between the graphite plates was 4.5 cm and the applied voltage was 15 V the best exfoliation efficiency was obtained. In order to identify the crucial parameters that dominate the whole process, further experiments were carried out. We fixed the electrolyte formula, followed by adjusting the voltage and electrolysis area. All of the data and results are presented in Table 2. From Table 2, we can obviously observe that the current density is a key factor in the exfoliation process, where 0.11 A cm−2 is a critical point. When the current density is less than 0.11 A cm−2, the exfoliation effect is not significant because the silver-like substances are produced slowly, which makes the process very time-consuming. On the other hand, when the current density is greater than 0.11 A cm−2, intense reaction will produce numerous silver-like substances. However, extreme exfoliation usually results in the production of relatively large graphite fragments, which will attach to the silver-like substances and result in inconsistent graphene quality. After 8 hours of electrochemical exfoliation, including water washing and sonication, we found that the production of graphene is around 0.335 g.
| Experimental parameters | |||
|---|---|---|---|
| Voltage (V) | 12 | 15* | 18 |
| Concentration of KOH (wt%) | 30* | 35 | 45 |
| Concentration of H2SO4 (wt%) | — | 13.5* | 23.8 |
| Distance (cm) | 2.5 | 3.5 | 4.5* |
| Voltage (V) | Current (A) | Electrode area (cm2) | Current density (A cm−2) | Results and notes |
|---|---|---|---|---|
| 6 | 0.84 | 35.2 | 0.05973 | Silver-like substances emerged after a long time |
| 9 | 1.80 | 35.2 | 0.04290 | Almost nothing exfoliated |
| 9 | 6.38 | 106.8 | 0.05114 | Silver-like substances were quite rare |
| 12 | 6.90 | 85.1 | 0.08108 | Silver-like substances existed |
| 15 | 9.49 | 85.1 | 0.11328 | Large amounts of silver-like substances |
| 18 | 12.02 | 89.38 | 0.13448 | Lots of black and silver-like substances exfoliated |
Table 3 displays a comparison of related literature on the electrochemical exfoliation of graphene, including the electrochemical procedure, electrolyte and other important parameters. Among all of the references, our materials exhibit the best quality, which can be inferred from having the lowest D/G ratio (around 0.061) based on Raman spectrometry.
| Electrochemical procedures | Electrolytes | Products | I D/IG ratio | O/C ratio | References |
|---|---|---|---|---|---|
| Anodic oxidation | NaOH + H2O2(aq.) | GNS | 0.67 | 0.06 | 19 |
| Anodic oxidation | PSS(aq.) | GNS | 0.6 | — | 20 |
| Anodic oxidation | TBA(aq.) | GNS | 0.64 | 0.09 | 21 |
| Anodic oxidation | Ionic liquid(aq.) | GNS | 0.1 | High | 22 |
| Cathodic reduction | NaCl(aq.) + DMSO + thin ion | GNS | 0.1 | 0.08 | 23 |
| Cathodic reduction | HClO4(aq.) | GNS | 0.1 | — | 24 |
| Cathodic reduction | PC | GNS | <0.1 | — | 25 |
| Anodic oxidation then cathodic reduction | H2SO4(aq.) | GNS | 0.6 | — | 9 |
| Anodic oxidation then cathodic reduction | H2SO4(aq.) or H3PO4(aq.) | GO | 0.71 | — | 26 |
| Anodic oxidation then cathodic reduction | Ionic liquid(aq.) | GNS and GO | 0.3 (GN), 0.75 (GO) | — | 27 |
| Anodic oxidation then cathodic reduction | H2SO4(aq.) + KOH(aq.) | GNS | 0.061 | — | This study |
2.2. Sample characterization
Field emission scanning electron microscopy (SEM, Hitachi S-4100), high resolution transmission electron microscopy (HR-TEM JOEL-2000FX), atomic force microscopy (AFM, Bruker ICON2-SYS), Raman spectrometry (Horiba iHR320, Olympus BX41, 532 nm excitation laser), X-ray diffraction (XRD, Bruker D8 Avance), Fourier transform infrared spectroscopy (FT-IR, Bruker Tensor-II) and X-ray photoelectron spectroscopy (XPS Thermo K-alpha) were carried out for functional group identification.3. Results and discussion
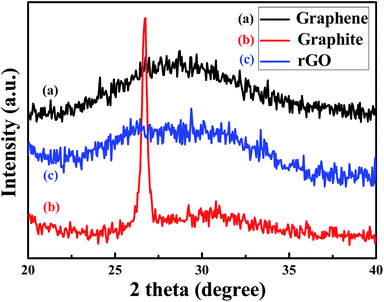
Fig. 2 shows the XRD patterns of EC-graphene (black), graphite (red) and rGO (blue). It is clearly shown that graphite has a high intensity characteristic peak at 26°. However, for graphene, this characteristic peak at 26° disappears, and is replaced by a broad and weak mound, showing that, no matter whether we utilize electrochemical or chemical approaches, well-exfoliated graphene can be obtained.
Fig. 3(a) shows the FT-IR spectra of EC-graphene and rGO produced using Hummers’ method after 300 °C thermal treatment. The weak peaks at 2927 and 2947 cm−1 indicate the asymmetric and symmetric vibrations of C–H groups. The appearance of peaks at 1674 cm−1 in both EC-graphene and rGO is attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups. It can be observed clearly that the peak is stronger for the former than the latter, which implies that GNSs possess more C
C groups. It can be observed clearly that the peak is stronger for the former than the latter, which implies that GNSs possess more C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups. There are peaks at 1299 and 1312 cm−1, which are attributed to the stretching vibration of C–O groups, and a weak peak for the GNSs at 1263 cm−1 can be ascribed to C–O–C groups. The existence of a weak peak at 1510 cm−1 is related to C–C stretching in the aromatic ring, which reveals that hexagonal carbon structures were detected. However, the low intensity of the peak also revealed that C–C groups were quite few in EC-graphene. FT-IR results confirm the presence of oxygen-containing functional groups in both EC-graphene and rGO. To further investigate the evidence of their formation, XPS analysis was implemented, as shown in Fig. 3(b). The presence of the XPS C1s binding energy at 284.1 eV is due to C
C groups. There are peaks at 1299 and 1312 cm−1, which are attributed to the stretching vibration of C–O groups, and a weak peak for the GNSs at 1263 cm−1 can be ascribed to C–O–C groups. The existence of a weak peak at 1510 cm−1 is related to C–C stretching in the aromatic ring, which reveals that hexagonal carbon structures were detected. However, the low intensity of the peak also revealed that C–C groups were quite few in EC-graphene. FT-IR results confirm the presence of oxygen-containing functional groups in both EC-graphene and rGO. To further investigate the evidence of their formation, XPS analysis was implemented, as shown in Fig. 3(b). The presence of the XPS C1s binding energy at 284.1 eV is due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C functionalities with sp2 carbon. Oxygen functional groups, such as C–O and COOH at the binding energies of 285.8 eV and 288.6 eV, can also be observed. The XPS survey spectra of C and O revealed the relative percentage of carbon and oxygen is 89.64% carbon and 10.36% oxygen, respectively. From FT-IR and XPS analysis, we can confirm that, compared to rGO, EC-graphene possesses more C
C functionalities with sp2 carbon. Oxygen functional groups, such as C–O and COOH at the binding energies of 285.8 eV and 288.6 eV, can also be observed. The XPS survey spectra of C and O revealed the relative percentage of carbon and oxygen is 89.64% carbon and 10.36% oxygen, respectively. From FT-IR and XPS analysis, we can confirm that, compared to rGO, EC-graphene possesses more C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups and fewer oxygen groups.
C groups and fewer oxygen groups.
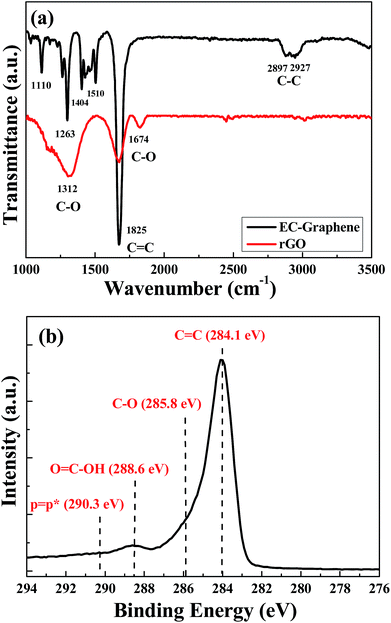 | ||
| Fig. 3 (a) Fourier transform infrared spectra of EC-graphene and rGO. (b) X-ray photoelectron spectrum of EC-graphene. | ||
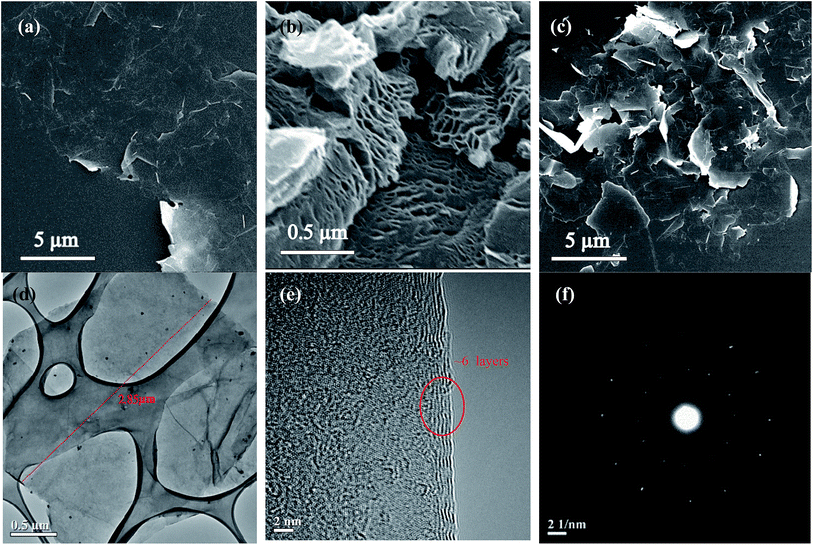
Fig. 4 shows the surface morphologies of EC-graphene and rGO for comparison. Fig. 4(a) shows the SEM image of electrochemically exfoliated graphene. As we can see, the surface of the graphene is quite smooth, flat and clear. The color of the silicon substrate in the bottom left-hand corner is almost identical to that of the samples, indicating that highly transparent few-layer graphene was obtained. In contrast, reduced GO (rGO), which was reduced under 1000 °C thermal treatment and manufactured using chemical exfoliation based on Hummers’ method, exhibits a porous block structure, which is quite different from that of the former and is shown in Fig. 4(b). The common point of both samples is the existence of little wrinkles and curling at the border. The reason for this phenomenon is that graphene itself tries to maintain a stable state thermodynamically and oxygen functional groups are present around the edge. Fig. 4(c) shows a SEM image of sediments in the electro-bath. We can observe that, even if the sample has a flaky structure, the thickness of as-synthesized sample is too thick. The thickness distribution is also inferior to that of the samples in Fig. 4(a). This is the reason that we only collected the upper floating silver substances, in order to maintain the quality of the graphene. Fig. 4(d) shows a TEM image of the sample on a Cu-grid. The GNS is nearly transparent and shows a uniform thickness distribution, with a lateral size of about 2.85 μm. The HR-TEM image in Fig. 4(e) shows that the edge of the suspended film always folds back and the ordered lattice is clearly observed. The image demonstrates that the thickness of EC-graphene was about 6 layers. In addition, a selected area electron diffraction (SAED) pattern is shown in Fig. 4(e). The well-defined diffraction spots prove the crystalline structure and low level of defects inside EC-graphene.
Raman spectroscopy is a convenient and non-destructive approach to examine the distortion and degree of defects in graphite-based materials. As we know, two characteristic peaks, generally named D (∼1355 cm−1) and G (∼1580 cm−1) bands, are observed in graphite-based materials. The G band represents the first order scattering of the E2g photons observed for the sp2 carbon domain, while the D band corresponds to the breathing mode or j-point photons of A1g symmetry, associated with the disorder band of a structural defect, amorphous carbon or an edge. The intensity ratio of the D band to G band (ID/IG) is usually used as a measure of disorder. Fig. 5 shows Raman spectra (excited by 532 nm laser light) of electrochemically exfoliated graphene produced under different current densities in (a)–(c). It is evident that with increasing current density, the ID/IG ratio becomes stronger and stronger, which might be due to the destruction and damage of the graphitic structure under a large current density. The structural defects of the GNSs, therefore, were observed in the Raman spectra. By contrast, the ID/IG ratio of chemically exfoliated graphene is about 1.38, which is at least 10 times less than that of the former sample, as shown in Fig. 5(e). From this analysis, we can reasonably assert that electrochemically produced EC-graphene is of higher quality than rGO.
 | ||
| Fig. 5 Raman spectra of EC-graphene produced under different current densities: (a) 0.05 A cm−2, (b) 0.08 A cm−2, and (c) 0.11 A cm−2; as well as (d) graphite and (e) rGO. | ||
Fig. 6(a) shows typical AFM images for GNS drop-cast on a silicon wafer; flakes with almost identical thickness can be observed. Here, we randomly chose a line-scan for the sample, as shown in Fig. 6(b). Apparently, the average thickness of the sample is around 2 nm. The statistical thickness distribution for the sample, shown in Fig. 6(c), indicates that most of the GNSs we obtained were 2–3 nm thick, which is around 6 layers of graphene. This result is consistent with the TEM images shown in Fig. 4(e). In light of the AFM analysis, we firmly believe that few-layer graphene can be obtained easily using this electrochemical method.
 | ||
| Fig. 6 AFM image of (a) drop-cast EC-graphene, (b) the line-scan and (c) the thickness distribution of EC-graphene. | ||
4. Conclusions
Taking quality and production cost into account, and in order to retain its structural integrity, the preferred route for the future manufacture of graphene is to directly exfoliate graphite into graphene. Electrochemical exfoliation is one of the methods that matches these criteria. Through a series of experiments, we found that the best exfoliation efficiency was achieved when the current density was around 0.11 A cm−2, after which detailed analysis was carried out. Morphological analysis using SEM and TEM showed that the electrochemically-exfoliated graphene had a uniform thickness distribution and smooth surface. The AFM analysis demonstrated the thickness of the EC-graphene to be around 2–3 nm, which indicated that around 6 layers of graphene were obtained. This result is in accordance with the HR-TEM analysis. In the Raman spectra, the D/G ratio of EC-graphene was only 0.061, which is 11 times less than that of rGO and showed that EC-graphene has barely any defects. FT-IR revealed different oxygen functional groups on EC-graphene and rGO. XPS gave further information about EC-graphene, in terms of the relative percentages of carbon and oxygen on the samples, and also verified the Raman spectroscopy findings. By using this electrochemical exfoliation method, high quality graphene has been produced that could be applied in a considerable number of applications in the future.Acknowledgements
I deeply appreciate the instruction from Professor Ching-Yuan Su and Lecturer Gui-Ting Xu for the experimental techniques and my professor Wei-Ren Liu for experimental guidance. Financial support was received from National Science Council of Taiwan and National Chung-Shan Institute of Science & Technology under contract no. NSC-101-2218-E-033-001 and NCSIST-0497-V109, respectively.Notes and references
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett., 2008, 8, 902 CrossRef CAS PubMed.
- K. I. Bolotin, K. J. Sikes, Z. Jiang, M. Klima, G. Fudenberg, J. Hone, P. Kim and H. L. Stormer, Solid State Commun., 2008, 146, 351 CrossRef CAS.
- X. Li, Y. Zhu, W. Cai, M. Borysiak, B. Han, D. Chen, R. D. Piner, L. Colombo and R. S. Ruoff, Nano Lett., 2009, 9, 4359 CrossRef CAS PubMed.
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451 CrossRef CAS PubMed.
- C. Berger, Z. Song, X. Li, X. Wu, N. Brown, C. Naud, D. Mayou, T. Li, J. Hass, A. N. Marchenkov, E. H. Conrod, P. N. First and W. A. de Heer, Science, 2006, 312, 1191 CrossRef CAS PubMed.
- X. Li, W. Cai, J. An, S. Kim, J. Nah, D. Yang and R. Piner, Science, 2009, 324, 1312 CrossRef CAS PubMed.
- X. S. Li, W. W. Cai, J. H. An, S. Kim, J. Nah, D. X. Yang, R. Piner, A. Velamakanni, I. Jung, E. Tutuc, S. K. Banerjee, L. Colombo and R. S. Ruoff, Science, 2009, 324, 1312 CrossRef CAS PubMed.
- C. Y. Su, A. Y. Lu, C. Y. Wu, Y. T. Li, K. K. Liu, W. J. Zhang, S. Y. Lin, Z. Y. Juang, Y. L. Zhong, F. R. Chen and L. J. Li, Nano Lett., 2011, 11, 3612 CrossRef CAS PubMed.
- C. H. Chen, C. T. Lin, Y. H. Lee, K. K. Liu, C. Y. Su, W. Zhang and L. J. Li, Small, 2011, 8, 43 CrossRef PubMed.
- C. Valles, C. Drummond, H. Saadaoui, C. A. Furtado, M. He, O. Roubeau, L. Ortolani, M. Monthioux and A. Penicaud, J. Am. Chem. Soc., 2008, 130, 15820 CrossRef PubMed.
- W. S. Hummers, US Pat. 798 (2):878, 1957.
- H. A. Becerril, J. Mao, Z. Liu, R. M. Stoltenberg, Z. Bao and Y. Chen, ACS Nano, 2008, 2, 463 CrossRef CAS PubMed.
- G. Eda, G. Fanchini and M. Chhowalla, Nat. Nanotechnol., 2008, 3, 270 CrossRef CAS PubMed.
- B. Z. Jang and A. Zhamu, J. Mater. Sci., 2008, 43, 5092 CrossRef CAS.
- P. Tripathi, Ch. R. P. Patel, M. A. Shaz and O. N. Srivastava, Graphene Times, 2013 Search PubMed.
- F. Kang, Y. Leng and T. Y. Zhang, J. Phys. Chem. Solids, 1996, 57, 889 CrossRef CAS.
- R. A. Greinke and R. A. Reynolds, US Pat. 416815 B2, 2002.
- K. Sanjeeva Rao, J. Senthilnathan, Y. F. Liu and M. Yoshimura, Sci. Rep., 2014, 4, 4237 Search PubMed.
- G. X. Wang, B. Wang, J. Park, Y. Wang, B. Sun and J. Yao, Carbon, 2009, 47, 3242 CrossRef CAS.
- A. J. Cooper, N. R. Wilson, I. A. Kinloch and R. A. W. Dryfe, Carbon, 2013, 66, 340 CrossRef.
- X. You, J. H. Chang, B. K. Ju and J. J. Pak, Nanosci. Nanotechnol., 2011, 11, 5965 CrossRef CAS.
- M. Zhou, J. Tang, Q. Cheng, G. Xu, P. Cui and L. C. Qin, Chem. Phys. Lett., 2013, 572, 61 CrossRef CAS.
- G. M. Morales, P. Schifani, G. Ellis, C. Ballesteros, G. Martínez, C. Barbero and H. J. Salavagione, Carbon, 2011, 49, 2809 CrossRef CAS.
- J. Z. Wang, K. K. Manga, Q. L. Bao and K. P. Loh, J. Am. Chem. Soc., 2011, 133, 8888 CrossRef CAS PubMed.
- J. Liu, H. Yang, S. G. Zhen, C. K. Poh, A. Chaurasia, J. Luo, X. Wu, E. K. L. Yeow, N. G. Sahoo, J. Lin and Z. Shen, RSC Adv., 2013, 3, 11745 RSC.
- V. V. Singh, G. Gupta, A. Batra, A. K. Nigam, M. Boopathi, P. K. Gutch, B. K. Tripathi, A. Srivastava, M. Samuel, G. S. Agarwal, B. Singh and R. Vijayaraghavan, Adv. Funct. Mater., 2012, 22, 2352 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |