Novel halogen-free flame retardants based on adamantane for polycarbonate
Abstract
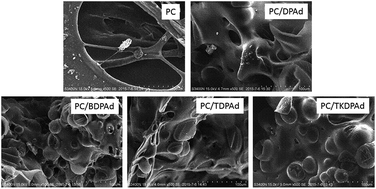
A novel series of flame retardants (FRs) containing phosphate moieties attached to a bridgehead-substituted adamantane, 1-(diphenyl phosphate) adamantane (DPAd), 1,3-bis(diphenyl phosphate) adamantane (BDPAd), 1,3,5-tris(diphenyl phosphate) adamantane (TDPAd) and 1,3,5,7-tetrakis(diphenyl phosphate) adamantane (TKDPAd), were systematically synthesized in an attempt to develop efficient FRs for polycarbonate (PC). Their chemical structures were confirmed by Fourier Transform Infrared Spectroscopy (FTIR), Nuclear Magnetic Resonance (1H and 31P NMR), elemental analysis and melting point measurements. The flame-retarding efficiencies of the FRs were evaluated by Limited Oxygen Index (LOI), UL-94 vertical burning experiments, Scanning Electron Microscopy (SEM), Cone Calorimeter Test (CCT), Thermogravimetric Analysis (TGA), FTIR and TGA-FTIR. For TKDPAd, thermal decomposition took place in a sharply two-step mechanism at high temperature above 381.5 °C. A significant improvement of flame-retardant performance in PC/TKDPAd among the PC/FR was observed with an addition of 8 wt% TKDPAd in the presence of an anti-dripping agent (0.1 wt%). High thermal stability and phosphorus content of flame retardant are believed to be of great importance for efficient flame-retardant action of adamantane-based phosphates.

 Please wait while we load your content...
Please wait while we load your content...