Ionic liquid-supported benzyl azide: an efficient soluble scavenger for alkynes†
Abstract
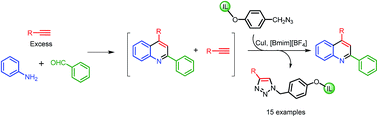
An ionic liquid functionalized with benzyl azide was synthesized and its synthetic utility was evaluated by scavenging excess alkynes in the synthesis of 2,4-disubstituted quinoline via the Povarov reaction. The ionic liquid-supported benzyl azide gave excellent efficiency in alkyne scavenging (85–100%). Purification of the products without column chromatography, ease of monitoring, high loading of the scavenger and shorter scavenging time are some of the advantages of this approach over solid-supported scavengers.

 Please wait while we load your content...
Please wait while we load your content...