Aligned carbon nanotube/polymer hybrid electrolytes for high performance dye sensitized solar cell applications†
Abstract
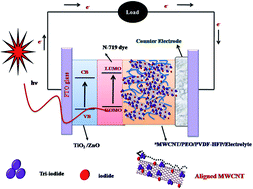
Novel multiwalled carbon nanotube (MWCNT) filled poly(ethylene oxide) (PEO) and poly(vinylidenefluoride-co-hexafluoropropylene) (PVDF-HFP) composite electrolytes were successfully prepared for solid state dye sensitized solar cell application. The prepared composite membranes were characterized by Fourier transform infrared (FTIR) spectroscopy, transmission electron microscopy (TEM), differential scanning calorimetry (DSC), thermogravimetry (TGA), UV-visible spectroscopy, electrochemical impedance spectroscopy (EIS) and linear steady state voltammetry (LSV) measurements. The incorporated MWCNTs were electrically aligned by an applied external DC electric field of 440 V cm−1. The alignment of MWCNTs in the PEO/PVDF-HFP matrix was confirmed by wide angle X-ray diffraction (WAXD) and Raman spectroscopy. The ionic conductivity doubled up to 5.72 mS cm−1 for aligned MWCNT/PEO/PVDF-HFP electrolyte in comparison to the electrolyte containing unaligned-MWCNT. It was observed from linear steady state voltammograms that the apparent diffusion coefficient was increased up to 7.1 × 10−9 cm2 s−1 (I−) for the electrically aligned MWCNT incorporated electrolyte. The polymer nanocomposites with the optimal composition (1 wt% of MWCNT in 4 : 6 wt% of PEO/PVDF-HFP) were used as an electrolyte for solid state dye sensitized solar cell (DSSC) fabrication. The DSSC with electrically aligned MWCNT/PEO/PVDF-HFP electrolyte showed a higher photoconversion efficiency of about 4% under the illumination of 100 mW cm−2, while the device based on unaligned MWCNT based electrolyte gave 3.2%. The enhancement was also confirmed by the electrochemical impedance spectra analyses of the DSSCs, which showed lower diffusion resistance (Rdif) and charge transfer resistance (Rct). The stability of the device was studied using chronoamperometry technique under several on–off cycles.

 Please wait while we load your content...
Please wait while we load your content...