Shape evolution of parallelogrammic magnesium oxalate controlled by phosphate species†
Abstract
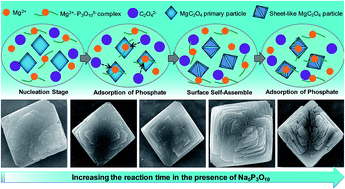
Phosphate species are capable of playing a crucial role in manipulating the shapes and properties of inorganic materials. Herein, we examined the shape evolution of magnesium oxalate dihydrate (MgC2O4·2H2O) to parallelogrammic microparticles with sheet-like structure in a precipitation process under the influence of phosphate species in the form of sodium tripolyphosphate (Na5P3O10). Supported by a series of time-dependent experiments, the shape evolution of MgC2O4·2H2O was evidenced by the complexation and blocking effect of Na5P3O10 via a later adsorption at the surface of the formed MgC2O4·2H2O product. The results from scanning electron microscopy (SEM) images, energy dispersive spectroscopy (EDS) and X-ray photoelectron spectrometry (XPS) measurements show that the shape evolution of parallelogrammic particles is accompanied by a modification in the chemical composition via a later adsorption. Our results demonstrated that the amount of Na5P3O10 participating into the self-assembly of the layer-like parallelogram varied with reaction time. In the initial reaction stage, little amount of Na5P3O10 is involved in the particle formation, and the participating amount increased with extent of reaction. This investigation presents the effect of Na5P3O10 on the self-assembly of MgC2O4·2H2O particles with layer-like structure and highlights its role in the controllable synthesis of microparticles.

 Please wait while we load your content...
Please wait while we load your content...