Promoting effects of MgO and Pd modification on the catalytic performance of hierarchical porous ZSM-5 for catalyzing benzene alkylation with methanol
Hualei Hu,
Jinghui Lyu,
Jie Cen,
Qunfeng Zhang,
Qingtao Wang,
Wenwen Han,
Jiayao Rui and
Xiaonian Li*
Industrial Catalysis Institute of Zhejiang University of Technology, State Key Laboratory Breeding Base of Green Chemistry Synthesis Technology, Hangzhou, 310032, P. R. China. E-mail: xnli@zjut.edu.cn
First published on 16th July 2015
Abstract
The effect of MgO and Pd modification on the catalytic performance of hierarchical porous ZSM-5 for benzene alkylation with methanol was investigated. The results indicated that the introduction of MgO could reduce the Brönsted acid sites which suppressed the side reaction of methanol to olefins and in turn effectively promoted the alkylation of benzene. However, the single modification of MgO could not completely suppress the formation of ethylbenzene and coke. Doping a small amount of Pd had a positive effect on inhibiting the generation of ethylbenzene and coke, which could be attributed to the hydrogenation of ethylene into ethane on Pd. The dual modified catalyst (MgO and Pd) exhibited high benzene conversion (56%) and xylene selectivity (39.1%), and the lowest ethylbenzene selectivity (0.13%) and coke content (0.4 wt%).
1. Introduction
The alkylation of benzene with simple alcohols to produce high value-added toluene and xylene is of great commercial value.1–3 Studies showed that hierarchical porous ZSM-5 could overcome the limitation of micropores to achieve better catalytic performance.4–6 However, suppressing the side reaction of methanol to olefins remains a challenge.5According to the literature, only a small amount of Brönsted acid sites was necessary for the alkylation of benzene and the catalyst with highly reduced Brönsted acidity could minimize the occurrence of the side reaction of methanol.7,8 The introduction of an additive compound into the zeolite system was found to be an effective method to adjust the acidity of ZSM-5.9 For example, the modification of ZSM-5 with SiO2 could efficiently eliminate external surface acid sites, and the introduction of zinc into ZSM-5 could form ZnOH+ (Lewis acid) at the expense of Brönsted acid.10–12 It should be noted that the formed ZnOH+ also could catalyze the dehydrogenation of light hydrocarbons into olefins which resulted in the formation of coke.12 Recently, Ding et al. had reported that the modification of mesopores ZSM-5 with Mg could effectively decrease the ratio of Brönsted acid to Lewis acid, which in turn improved the conversion of benzene in the alkylation of benzene with ethanol.13 This prompted us to investigate the effect of MgO modification on the performance of hierarchical porous ZSM-5 in the alkylation of benzene with methanol.
Meanwhile, previous researches pointed out that the ethylene, which was the main constituent in olefins, would lead to the formation of ethylbenzene by the alkylation of benzene with ethylene and the generation of coke via the polymerization of ethylene with itself.4,9 Moreover, the separation of ethylbenzene from xylene was difficult and the carbon deposition could cause the deactivation of catalyst.9,14 Our previous work indicated that the modification of ZSM-5 with Pt could effectively suppress the formation of ethylbenzene and coke by the hydrogenation of ethylene into ethane.4 Pd has been found to be more active for ethylene hydrogenation with lower cost.15 The substitution of Pt by Pd would help with the suppression of ethylbenzene and further decrease the cost of xylene production.
In present work, the catalytic performance of MgO and Pd modified hierarchical porous ZSM-5 in benzene alkylation with methanol was investigated. The textural properties and acidity of the catalysts were systematically characterized by various techniques (including XRD, BET, NH3-TPD, Py-IR and TG), and the conversion of benzene and the selectivity to products versus MgO and Pd content were also investigated. We observed that the modification of appropriate MgO content could suppress the side reaction of methanol to olefins by reducing the Brönsted acidity of catalyst. However, further inhibiting the formation of ethylbenzene and coke on MgO modified catalyst should depend on the doping of Pd.
2. Experimental
2.1. Catalyst preparation
Hierarchical porous ZSM-5 was prepared via solvent evaporation assisted dry-gel conversion route.16 In a typical synthesis, a homogeneous solution was first mixed from tetraethylorthosilicate (TEOS, Shanghai Chemical Reagent Co.), aluminum isopropoxide (AIP, Sinopharm Chemical Reagent Co., Ltd.), tetra-n-propylammonium hydroxide (TPAOH, Aladdin Industrial Inc.), hexadecyltrimethoxysilane (HTS, Aladdin Industrial Inc.) and ethanol (EtOH, Anhui Ante Food Company Co., Ltd.) with a molar ratio of 1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0028Al2O3
0.0028Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2TPAOH
0.2TPAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05HTS
0.05HTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15EtOH. The resultant gel mixture was aged at room temperature for 24 h and placed into a PTFE cup before transferring into a Teflon-lined autoclave. A small amount of water was added outside the PTFE cup to create steam for the hydrothermal synthesis conditions. Then, the stainless steel autoclave was heated at 180 °C for 72 h. After crystallization, the product was obtained by filtration without ion-exchange steps and calcined at 550 °C for 7 h (heating rate of 10 °C min−1) to obtain H-form hierarchical porous ZSM-5.
15EtOH. The resultant gel mixture was aged at room temperature for 24 h and placed into a PTFE cup before transferring into a Teflon-lined autoclave. A small amount of water was added outside the PTFE cup to create steam for the hydrothermal synthesis conditions. Then, the stainless steel autoclave was heated at 180 °C for 72 h. After crystallization, the product was obtained by filtration without ion-exchange steps and calcined at 550 °C for 7 h (heating rate of 10 °C min−1) to obtain H-form hierarchical porous ZSM-5.
MgO modified hierarchical porous ZSM-5 catalysts with a nominal MgO loading of 1 to 5 wt% were prepared by impregnating method. Firstly, the synthesized hierarchical porous ZSM-5 was tableted, crushed and screened to the particles with diameters of 0.45–0.90 mm. The sample was then impregnated with quantitative amounts of aqueous solution of Mg(NO3)2·6H2O at ambient temperature for 24 h before dried at 110 °C overnight and calcined at 550 °C for 7 h. Pd modified hierarchical porous ZSM-5 catalyst was prepared by impregnating catalyst with aqueous chloropalladic acid at ambient temperature for 24 h, followed by drying at 110 °C for 2 h and calcined again at 550 °C for 7 h.
The dual modified catalyst (MgO and Pd) was prepared by impregnating method. Firstly, the synthesized hierarchical porous ZSM-5 (0.45–0.90 mm) was impregnated with quantitative amounts of aqueous solution of Mg(NO3)2·6H2O at ambient temperature for 24 h before dried at 110 °C overnight and calcined at 550 °C for 7 h. The MgO modified catalyst was then impregnated with aqueous chloropalladic acid at ambient temperature for 24 h, followed by drying at 110 °C for 2 h and calcined again at 550 °C for 7 h.
2.2. Catalyst characterization
X-ray diffraction (XRD) (SCINTAG X′′ TRA) was measured using Cu K-radiation (1542 A) at 30 mA and 40 kV with scanning angle (2θ) from 5 to 80°. The nitrogen sorption isotherms were conducted at 77 K (ASAP-2020, Micromeritics). The specific surface area of the sample was evaluated using the Brunauer–Emmett–Teller (BET) method. The differential thermal analysis (TG-DTA) was evaluated by Netzsch STA 449C apparatus using a temperature ramp from 30 to 800 °C with a heating rate of 10 °C min−1 in oxygen atmosphere. NH3 temperature-programmed desorption (TPD) of the catalysts was carried out using a Tianjin XQ TP-5076 chemisorption instrument equipped with thermal conductivity detector (TCD). The sample was evacuated at 400 °C for 1 h in the flow of He and then cooled down to 50 °C. The adsorption of NH3 was performed with the flow of 10 vol% NH3/Ar at 50 °C. The desorption was conducted between 50 and 650 °C with a heating rate of 10 °C min−1. The TCD signals were calibrated using 10 μL of NH3 as standard. Fourier transform infrared (FT-IR) spectra were measured on a Nexus FT-IR spectrometer. The sample was firstly pressed into a wafer, and then heat-treated in He flow (20 mL min−1) at 400 °C for 1 h. IR spectra were recorded at room temperature. For pyridine adsorption (Py-IR), pyridine vapour was introduced into the cell at room temperature for 1 h; the spectra were then recorded after evacuation at 200 °C and 400 °C for 1 h, respectively. The concentrations of Brönsted acid (B) and Lewis acid (L) were calculated from the peak areas of adsorbed pyridine at around 1540 and 1450 cm−1, and the extinction coefficients of ε(B) and ε(L) was 1.88 and 1.42 cm mmol−1, respectively.172.3. Catalytic activity test
All evaluation experiments were carried out in a continuous-flow fixed-bed reactor with a stainless steel tube (8 mm i.d.) at atmospheric pressure. In each test, 0.5 g catalyst diluted with 5.0 g inert quartz sand were loaded in the middle of the tube reactor. In order to investigate the effect of carrier gas on the catalytic performance of catalyst, the catalyst with the same content of ZnO was divided into two parts and tested in hydrogen atmosphere and nitrogen atmosphere, respectively. The catalyst was heat-treated in situ from ambient temperature to 400 °C at 10 °C min−1 and maintained at 400 °C for 1 h in H2 flow with a space velocity of 2400 h−1. Then benzene and methanol mixture (B/M molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was fed into the reactor (WHSV = 2.0 h−1) at 400 °C with a co-feed H2 flow of 40 mL min−1. The effluent from the reactor was analyzed online by the gas chromatography (Fuli GC9790) with a DB-1capillary column (30 m × 0.25 mm × 1.00 μm) and a flame ionization detector. In order to ensure all of the products were in gas phase, the temperature of the effluent line was maintained at 200 °C by heating belt.
1) was fed into the reactor (WHSV = 2.0 h−1) at 400 °C with a co-feed H2 flow of 40 mL min−1. The effluent from the reactor was analyzed online by the gas chromatography (Fuli GC9790) with a DB-1capillary column (30 m × 0.25 mm × 1.00 μm) and a flame ionization detector. In order to ensure all of the products were in gas phase, the temperature of the effluent line was maintained at 200 °C by heating belt.
3. Results and discussion
3.1. Catalyst characterization and tests
Fig. 1 showed the XRD patterns of unmodified and modified hierarchical porous ZSM-5 catalysts. It can be seen that all the samples exhibited similar characteristic diffraction peaks of MFI structure, indicating that the crystals of hierarchical porous ZSM-5 were preserved during the modification. In addition, no patterns corresponding to MgO and Pd were detected in the XRD spectra, suggesting that MgO and Pd might be highly dispersed on the surface of ZSM-5.10,13,18The SEM images in Fig. 2a and b show the crystal morphology of synthesized hierarchical porous ZSM-5 zeolites, it can be seen that the shape of hierarchical ZSM-5 crystals is roughly ellipsoidal with a size of about 200–300 nm. The high-magnification TEM image (Fig. 2c) indicated that the aggregates were made of nanoparticles ranging from 10 to 50 nm. Further zooming the image, we could see that the nanoparticles were crystalline in nature (Fig. 2d). These phenomenons were consistent with the literature reported by Zhu et al.16 The N2 physisorption isotherms of synthesized hierarchical porous ZSM-5 are given in Fig. 3. The samples exhibited the characteristic features of type-IV isotherm, an obvious hysteresis loop which is associated with capillary condensation taking place in mesopores and the limiting uptake over a range of high p/p0. Through the BJH method, the pore size distribution of hierarchical porous ZSM-5 was obtained as shown in the Fig. 3 inset. The presence of mesopores could be clearly observed. Based on the discussion above, the hierarchical porous ZSM-5 was successful synthesized in present work.
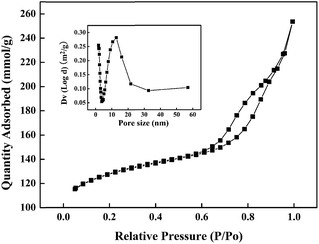 | ||
| Fig. 3 Nitrogen adsorption–desorption isotherms of synthesized hierarchical porous ZSM-5 and the pore-size distribution deduced from the adsorption branch of the isotherm is shown in the inset. | ||
According to Table 1, besides a micropore volume of 0.13 cm3 g−1, an additional mesopore volume of 0.28 cm3 g−1 for hierarchical porous ZSM-5 was observed. With the modification of MgO, the BET surface area and pore volume were continuously decreased. The decrease of micropore volume might be attributed to the micropore of zeolite was filled by magnesium oxide.19 We noted that the mesopore volume was also decreased, which suggesting that part of MgO particles was dispersed in the mesopores. As compared to MgO, a small amount of Pd introduced into hierarchical porous ZSM-5 did not exhibit significant impact on the BET surface area.
| Catalyst | SBET (m2 g−1) | Vmicroa (cm3 g−1) | Vmesob (cm3 g−1) |
|---|---|---|---|
| a Micropore volume is estimated using the t-plot method.b Mesopore macropore volume is calculated by a subtraction of total pore volume at a relative pressure of P/P0 = 0.99 from the micropore pore volume obtained from the t-plot method. | |||
| 0 wt% MgO/ZSM-5 | 408 | 0.128 | 0.28 |
| 1 wt% MgO/ZSM-5 | 370 | 0.119 | 0.25 |
| 3 wt% MgO/ZSM-5 | 345 | 0.115 | 0.25 |
| 5 wt% MgO/ZSM-5 | 313 | 0.110 | 0.24 |
| 0.02 wt% Pd/ZSM-5 | 404 | 0.127 | 0.27 |
| 3 wt% MgO + 0.02 wt% Pd/ZSM-5 | 340 | 0.114 | 0.25 |
Fig. 4 showed the catalytic performance of unmodified and MgO modified hierarchical porous ZSM-5 in benzene alkylation with methanol. It can be seen that with the increase of MgO content from 0 to 3 wt%, the conversion of benzene and the selectivity to xylene gradually increased from 51.0 to 56.1% and 33.5–38.1%, respectively. Moreover, the selectivity to ethylbenzene decreased from 3.4 to 0.34%, indicating that the modification of hierarchical porous ZSM-5 could suppress the formation of ethylbenzene. Further increasing the content of MgO to 5 wt%, the conversion of benzene decreased to 55.4%. Therefore, 3 wt% of MgO was considered as the appropriate loading for the modification of hierarchical porous ZSM-5 catalyst. We also noted that the catalytic activity of MgO modified catalyst (56.1%) was higher than that of Zn modified catalyst (53.6%) which had reported in our previous work, indicating that the modification of MgO was with the better promoting effect. However, the selectivity to ethylbenzene of 3 wt% MgO/ZSM-5 (0.34%) was still need to be reduced.
 | ||
| Fig. 4 Catalytic performances of MgO modified hierarchical porous ZSM-5 in benzene alkylation with methanol. | ||
As shown in Fig. 5, with the modification of 0.02 wt% Pd, the selectivity to ethylbenzene decreased from 3.4 to 0.82% and the selectivity to xylene increased from 33.5 to 35.2%. It was worth noting that the conversion of benzene did not show big changes, indicating that the modification of Pd could not effectively promote the alkylation of benzene and this was similar with Pt modification. As for the catalyst modified with 3 wt% MgO and 0.02 wt% Pd, the conversion of benzene and the selectivity to xylene increased from 51.0 to 56.0% and 33.5 to 39.1%, respectively. We also noted that the selectivity to ethylbenzene decreased to 0.13%, which was lower than that of 3 wt% MgO modified ZSM-5 and 0.02 wt% Pd modified ZSM-5, strongly suggesting the promoting effect of MgO and Pd modification on the suppression of ethylbenzene formation in benzene alkylation with methanol. Although modifying the catalyst with 0.1 wt% Pt could decrease the selectivity of ethylbenzene to 0.15%, the cost of catalyst was increased while the conversion of benzene and selectivity of xylene was not increased.5 Obviously, the dual modified hierarchical porous ZSM-5 were with the better advantage both on the cost and production efficiency. Therefore, it was necessary to investigate the specific effect of MgO and Pd on the physical and chemical properties of catalyst.
 | ||
| Fig. 5 Catalytic performance of Pd and MgO modified hierarchical porous ZSM-5 in benzene alkylation with methanol. | ||
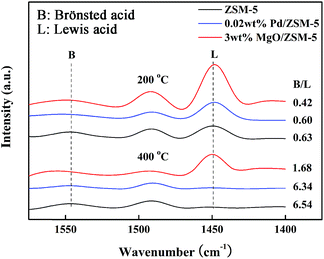
According to the literatures, when MgO was introduced into ZSM-5, the added Mg2+ would react with the protons of bridging hydroxyls to form Mg(OH)+ which would lead to the increase of the number of Lewis acid sites at the expense of the Brönsted acid sites.13,19 Thus, the introduction of MgO could effectively adjust the L/B acid proportion.13 In order to confirm the change of acid sites and acid strength of modified hierarchical porous ZSM-5, NH3-TPD (Fig. 6) and Py-IR (Fig. 7) were performed. Fig. 6 showed the NH3-TPD profiles of unmodified and modified hierarchical porous ZSM-5 catalysts. The amounts of ammonia desorbed from the catalyst surface can be observed via TPD peak areas. It can be observed that the hierarchical porous ZSM-5 exhibited two desorption peaks, which were labeled α and β, respectively. Peak α represents ammonia adsorbed on weak acid sites, and peak β represents ammonia adsorbed on strong acid site. As shown in Fig. 6, the NH3-TPD profile of Pd modified ZSM-5 was almost identical to that of unmodified ZSM-5, indicating that the acid strength of ZSM-5 was not influenced by the introduction of Pd. However, as for MgO modified catalyst, with the increase of MgO amount, the peak β was gradually disappeared while the total amount of NH3 absorbed was almost the same, which suggested that the amounts of strong acid sites of ZSM-5 were effectively modified and the Mg(OH)+ with the lower acidity was formed.
The pyridine-adsorbed IR spectra of modified hierarchical porous ZSM-5 catalysts were shown in Fig. 7. With the modification of MgO, the ratio of Brönsted acid sites to Lewis acid sites (expressed by B/L) was decreased and this was consistent with the result reported by Mao et al.19 While for Pd modified ZSM-5, the value of B/L was similar to that of unmodified ZSM-5. Yang et al. had reported that for the ZSM-5 loaded with high Pd content, large Pd particles could block the pores or cover the acid sites causing the decrease of acid sites.20 In our work, the content of Pd might be too small to cause any influence on the acidity of ZSM-5.
Adebajo et al. had reported that the catalyst with highly reduced Brönsted acidity could minimize the occurrence of the side reaction of methanol to olefins and enhance the methylation of benzene with methanol.7,8 In our previous work, we had observed that the modification of hierarchical porous ZSM-5 with Zn could suppress the reactions of methanol to olefins by reducing Brönsted acidity.12 Therefore, ethylbenzene on MgO modified catalyst might possibly be suppressed due to the same reason. However, the change of acidity was not responsible for the suppression of ethylbenzene on Pd modified catalyst due to that the acidity of modified catalyst was similar to that of unmodified one. Our previous work found that the mechanism of Pt to suppress ethylbenzene formation was the rapid hydrogenation of ethylene to ethane which avoided the alkylation of ethylene and benzene to form ethylbenzene.4 In order to verify the specific mechanism of MgO and Pd modification to suppress the formation of ethylbenzene, it was necessary to investigate the changes of the content of light hydrocarbons in the products. In addition, it has long been recognized that, ethylene, one main ingredient in light hydrocarbons, can form coke by polymerization.9 Therefore, the changes of coke should also be investigated.
As shown in Table 2, with the introduction of MgO, the total content of light hydrocarbons (C1–C3) obviously decreased. However, the opposite result was observed on Pd modified catalyst. The clear decrease and increase in the respective content of ethylene and ethane over Pd modified catalyst indicated that the ethylene was hydrogenated to ethane. Moreover, we noted that the content of ethylene on unmodified catalyst was 0.11 wt%, the increased amount of ethane on Pd modified catalyst (0.45 wt%) could not be achieved even all these ethylene were hydrogenated into ethane. Thus, the increased content of ethane should be related to the ethylene which alkylated with benzene to form ethylbenzene on unmodified catalyst. TG was performed to evaluate the coke content (removed within 200 to 600 °C) of the catalysts after 10 h usage. As seen in Fig. 8, it was clear that the coke content of MgO modified catalyst (0.8 wt%) was lower than that of unmodified catalyst (1.9 wt%). Considering the changes of total content of light hydrocarbons and coke, it was reasonable to conclude that the side reaction of methanol to olefins was suppressed on MgO modified catalyst and this in turn inhibited the formation of ethylbenzene. For Pd modified catalyst, the content of coke (1.1 wt%) was also lower than that of unmodified catalyst, indicating that the hydrogenation of ethylene could suppress the polymerization of ethylene to generate coke. It was worth noting that the catalyst 3 wt% MgO − 0.02 wt% Pd/ZSM-5 showed an apparent decrease in the content of coke (0.4 wt%), which was lower than that of either single modified catalyst (3 wt% MgO/ZSM-5 and 0.02 wt% Pd/ZSM-5). This indicated that the catalyst 3 wt% MgO − 0.02 wt% Pd/ZSM-5 could effectively combine the effect of MgO on the acidity which suppressed the side reaction of methanol to olefins and the ability of Pd on the hydrogenation of ethylene which avoided the polymerization of ethylene.
| Catalyst | Content (wt)/% | ||||
|---|---|---|---|---|---|
| Methane | Ethylene | Ethane | C3 | Total | |
| a Total: methane + ethylene + ethane + C3. | |||||
| Unmodified | 0.05 | 0.11 | 0.04 | 0.59 | 0.79 |
| 3 wt% MgO | 0.04 | 0.18 | 0.06 | 0.27 | 0.55 |
| 0.02 wt% Pd | 0.04 | 0.04 | 0.49 | 0.69 | 1.26 |
| 3 wt% MgO + 0.02 wt% Pd | 0.04 | 0.01 | 0.38 | 0.35 | 0.78 |
4. Conclusions
The appropriate modification method was developed for improving the catalytic performance of hierarchical porous ZSM-5 in catalyzing benzene alkylation with methanol. The suppression of the formation of ethylbenzene and coke could be achieved by the single modification of catalyst with MgO or Pd. However, the mechanism of MgO modification was to suppress the side reaction of methanol to olefins by the reduction of Brönsted acidity, and the introduction of Pd was to reduce the amount of ethylene by the hydrogenation of ethylene. The promoting effect of MgO and Pd modification on suppress the formation of ethylbenzene and coke was achieved on the dual modification catalyst.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (NSFC-21476207) and National Basic Research Program of China (973 Program) (No. 2011CB710800).References
- J. H. Ahn, R. Kolvenbach, S. S. Al-Khattaf, A. Jentys and J. A. Lercher, ACS Catal., 2013, 3, 817–825 CrossRef CAS.
- X. M. Wang, J. Xu, G. D. Qi, B. J. Li, C. Wang and F. Deng, J. Phys. Chem. C, 2013, 117, 4018–4023 CAS.
- W. Alabi, L. Atanda, R. Jermy and S. Al-Khattaf, Chem. Eng. J., 2012, 195–196, 276–288 CrossRef CAS PubMed.
- H. L. Hu, Q. F. Zhang, J. Cen and X. N. Li, Catal. Commun., 2014, 57, 129–133 CrossRef CAS PubMed.
- H. L. Hu, Q. F. Zhang, J. Cen and X. N. Li, Catal. Lett., 2015, 145, 715–722 CrossRef CAS.
- W. Deng, X. He, C. Zhang, Y. Gao, X. D. Zhu, K. K. Zhu, Q. S. Huo and Z. J. Zhou, Chin. J. Chem. Eng., 2014, 22, 921–929 CrossRef CAS PubMed.
- M. O. Adebajo, R. F. Howe and M. A. Long, Catal. Today, 2000, 63, 471–478 CrossRef CAS.
- M. O. Adebajo and M. A. Long, Catal. Commun., 2003, 4, 71–76 CrossRef CAS.
- Y. Zhao, W. Tan, H. Y. Wu, A. F. Zhang, M. Liu, G. M. Li, X. S. Wang, C. S. Song and X. W. Guo, Catal. Today, 2011, 160, 179–183 CrossRef CAS PubMed.
- W. Tan, M. Liu, Y. Zhao, K. K. Hou, H. Y. Wu, A. F. Zhang, H. Liu, Y. R. Wang, C. S. Song and X. W. Guo, Microporous Mesoporous Mater., 2014, 196, 18–30 CrossRef CAS PubMed.
- X. J. Niu, J. Gao, Q. Miao, M. Dong, G. F. Wang, W. B. Fan, Z. F. Qin and J. G. Wang, Microporous Mesoporous Mater., 2014, 197, 252–261 CrossRef CAS PubMed.
- H. L. Hu, J. H. Lyu, Q. T. Wang, Q. F. Zhang, J. Cen and X. N. Li, RSC Adv., 2015, 5, 32679–32684 RSC.
- W. Ding, Y. Y. Cui, J. J. Li, Y. Q. Yang and W. P. Fang, RSC Adv., 2014, 4, 50123–50129 RSC.
- F. Bauer, W. H. Chen, H. Ernst, S. J. Huang, A. Freyer and S. B. Liu, Microporous Mesoporous Mater., 2004, 72, 81–89 CrossRef CAS PubMed.
- A. K. Aboul-Gheit, S. A. Hanafy, A. A. Aboul-Enein and S. A. Ghoneim, J. Taiwan Inst. Chem. Eng., 2011, 42, 860–867 CrossRef CAS PubMed.
- K. K. Zhu, J. M. Sun, J. Liu, L. Q. Wang, H. Y. Wan, J. Z. Hu, Y. Wang, C. H. F. Peden and Z. M. Nie, ACS Catal., 2011, 1, 682–690 CrossRef CAS.
- C. A. Emeis, J. Catal., 1993, 141, 347–354 CrossRef CAS.
- A. Veses, B. Puértolas, M. S. Callén and T. García, Microporous Mesoporous Mater., 2015, 209, 189–196 CrossRef CAS PubMed.
- D. S. Mao, W. M. Yang, J. C. Xia, B. Zhang, Q. Y. Song and Q. L. Chen, J. Catal., 2005, 230, 140–149 CrossRef CAS PubMed.
- P. P. Yang, Y. C. Shang, J. Wang, J. F. Yu and T. H. Wu, React. Kinet. Catal. Lett., 2007, 91, 391–398 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |