Lithium doped polyaniline and its composites with LiFePO4 and LiMn2O4-prospective cathode active materials for environment friendly and flexible Li-ion battery applications
Abstract
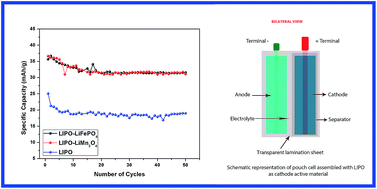
The present work is aimed at investigating the electrochemical properties of Li-ion cells, assembled using lithium substituted polyaniline and its composites with LiFePO4 and LiMn2O4 as the cathode active materials. Lithium substitution in PANI can be achieved by a variety of approaches and the present work introduces one of the cost effective methods using a comparatively cheaper lithium salt, n-butyllithium in hexanes (n-BuLi) as the dopant. X-ray diffraction, FTIR and SEM techniques were used to get the structural and surface morphological details of the Li-substituted PANI (LIPO) samples. Coin cells were assembled in the glove box using LIPO as cathode and lithium metal foil as anode with 1 M LiPF6 in EC : DMC (1 : 1) as electrolyte. Voltage sweep cyclic voltammetry and charge–discharge cycling were employed to characterize the electrochemical capabilities of the assembled cells. The cells are found to show a maximum discharge capacity of 25.04 mA h g−1 with 99.99% columbic efficiency and have got tremendous cycling stability over 50 cycles. Pouch cells were assembled using LIPO samples as cathode active materials, graphite as anode and gel polymer (poly(ethylene oxide)) as electrolyte. The pouch cells are found to hold the open circuit voltage of 0.6 V (OCV) even when bent up to 90° from the initial state, which provides the first signature of constructional flexibility of these polymer based Li ion cells. Hoping to improve the specific capacity of LIPO based cells, composite cathode materials were synthesized by mixing LIPO samples with LiFePO4 and LiMn2O4 in 9 : 1 ratio by weight. The cells are found to show a maximum discharge capacity of 37 mA h g−1 with 99.99% columbic efficiency and have got excellent cycling stability over 50 cycles. One of the highlights of these studies is the observation that the inherent Jahn–Teller effect associated with LiMn2O4 cathode material can be eliminated to a great extent by making composites with LIPO samples.

 Please wait while we load your content...
Please wait while we load your content...