Palladium salt and functional reduced graphene oxide complex: in situ preparation of a generally applicable catalyst for C–C coupling reactions†
Sheng Wanga,
Donghua Hub,
Wenwen Huab,
Jiangjiang Gua,
Qiuhong Zhanga,
Xudong Jia*a and
Kai Xi*b
aState Key Laboratory of Coordination Chemistry, Department of Polymer Science & Engineering, Nanjing National Laboratory of Microstructures, Nanjing University, Nanjing 210093, P. R. China. E-mail: jiaxd@nju.edu.cn; Fax: +86-25-83621337
bDepartment of Polymer Science & Engineering, Nanjing University, Nanjing 210093, P. R. China. E-mail: xikai@nju.edu.cn; Fax: +86-25-83686197
First published on 12th June 2015
Abstract
A novel Pd catalyst was designed by affording Pd2+ salt on the surface of functional reduced graphene oxide (FRGO), providing a new cheap and stable in situ prepared palladium catalyst for C–C coupling reactions, including the Heck reaction, Suzuki reaction, C–H bond functionalization reactions of thiophenes, and terminal alkyne C–H activation and homocoupling.
Many products could be synthesized by coupling reactions, either for the first time or in a much more efficient way than before.1–4 These reactions have largely been employed in academic laboratories as well as in pharmaceutical and fine chemical industries to synthesize a large variety of organic molecules.4–7 In these reactions, palladium catalysts have gained enormous relevance as the most commonly used catalysts.8,9 In the last decade, palladium nanoparticles have attracted more and more attention in the catalytic field.10,11 Compared to traditional catalysts, most nanosized catalysts have much higher efficiency.12 Several palladium nanosized catalysts for C–C bond formation reactions have been described in the literatures, but preparation of these catalysts is usually complicated, in a strict process.13–16
A common and critical feature of these catalytic processes is the formation of aryl or alkyl palladium(II) intermediates, which can be subsequently functionalized to form carbon–carbon and carbon–heteroatom bonds.17,18 Palladium(II) catalysts are sometimes used to replace palladium(0) catalysts which can be prepared from cheap and stable palladium salt such as PdCl2.19 We designed to composite palladium(II) salt and supporting materials for the preparation of palladium nanoparticles to absorb both of their merits. In this way, dispersion, size and stabilization problems in preparation of palladium nanoparticles can be abstained, and high-efficiency of palladium nanoparticle catalysts could be reserved. Many attempts have been made to immobilize Pd nanoparticles in a wide variety of supports.20–29 Among these supports, graphene oxide (GO) is an extremely versatile carbon material which can be modified by anchoring molecules with favorable properties.30,31
In our previous work, we had prepared an amino group FRGO and developed a single crystal triangle Au nanoplates supported by this FRGO, which showed high efficiency in reduction of 4-nitro phenol.32 As palladium–amine complex can form on FRGO in situ by direct coordination,18,33 we use the same FRGO dispersed in DMF to produce the catalyst, without extra ligand to afford palladium system. When the solution of palladium chloride in DMF was added into FRGO, the new catalyst was generated. This catalyst can be widely used in Suzuki–Miyaura reaction. In all the examples we have tried, the reactions are carried out mildly at room temperature with a low catalyst amount, and a high yield, moreover, unaffected in the presence of air. Meanwhile, Mizoroki–Heck reaction, C–H bond functionalization reactions of thiophenes, as well as terminal alkyne C–H activation leading to Glaser coupling can also be catalyzed. By this means, a new methodology in palladium catalyzed coupling reaction is developed.
GO was firstly prepared by modified Hummers method. The functionalized graphene oxide was obtained from reaction of 0.1 g of GO powders and 0.2 g of N-propylethane-1,2-diamine, then reduced by hydrazine hydrate. The as-synthesized FRGO (1 mL, 0.3 mg mL−1) and a DMF solution of PdCl2 (0.25 mL, 0.01 M) were mixed at room temperature. This catalyst was prepared and would be applied in reactions immediately (TEM images in Fig. 1a and b). The XPS result showed the palladium existed in divalent state. The palladium content of the obtained Pd(II)–FRGO was amounted to 4.78% by weight as verified by EDX, which could be converted into 0.59% by number of atom. Meanwhile, the amount of chloride was 0.60% by number of atom, equal to the amount of Pd practically (Fig. 1c). The particle size distribution of the Pd2+ nanoparticles on the FRGO supports was determined by TEM analysis, and the average particle diameter was 6.14 nm (Fig. 1d).
Then, this in situ prepared, cheap and stable palladium catalyst was applied to several series of C–C coupling reactions, which showed good catalytic performance and general applicability.
The palladium-catalyzed Suzuki–Miyaura reaction is one of the most widely used synthetic protocols in modern chemistry and in industrial application.34,35 These reactions were carried out with Pd(II)–FRGO, boronic acid (7a–7f, 8) (0.55 mmol, 1.1 equiv.), sodium carbonate (106 mg, 1 mmol, 2 equiv.), aryl bromide or iodide (1–6) (0.5 mmol, 1 equiv.), using ethanol (2 mL) and water (2 mL) as the solvent.13 The 26 coupling reactions proceeded smoothly to give the product with 84–97% yields under nitrogen in room temperature. Further experiments proved the reaction could even be carried out in atmosphere. To shorten the reaction time, we chose the catalyst in 0.25 mol% of reactant to control all the reaction time in less than 8 hours (Table 1).
| Entry | A | B | Yield (%) |
|---|---|---|---|
| a Structure of the products see in the ESI. | |||
| 1 | 1 | 7a | 9, 96 |
| 2 | 2 | 7a | 10a, 94 |
| 3 | 2 | 7b | 10b, 85 |
| 4 | 2 | 7c | 10c, 91 |
| 5 | 2 | 7d | 10d, 92 |
| 6 | 2 | 7e | 10e, 93 |
| 7 | 2 | 7f | 10f, 84 |
| 8 | 3 | 7a | 11a, 93 |
| 9 | 3 | 7b | 11b, 90 |
| 10 | 3 | 7c | 11c, 80 |
| 11 | 3 | 7e | 11d, 97 |
| 12 | 3 | 7f | 11e, 88 |
| 13 | 4 | 7a | 12a, 96 |
| 14 | 4 | 7b | 12b, 95 |
| 15 | 4 | 7c | 12c, 90 |
| 16 | 4 | 7d | 12d, 94 |
| 17 | 4 | 7e | 12e, 90 |
| 18 | 4 | 7f | 12f, 89 |
| 19 | 5 | 7a | 13a, 89 |
| 20 | 5 | 7b | 13b, 88 |
| 21 | 5 | 7c | 13c, 93 |
| 22 | 5 | 7d | 13d, 93 |
| 23 | 5 | 7e | 13e, 93 |
| 24 | 5 | 7f | 13f, 94 |
| 25 | 6 | 7d | 14a, 84 |
| 26 | 6 | 8 | 14b, 86 |
After the reaction, the catalyst was separated and characterized by EDX. Compared to the catalyst we prepared before the reaction, the amount of Pd increased to 1.00% by number of atom, with the chloride atom disappeared from the catalyst. In the reaction, more palladium ion from dissociative PdCl2 could be further fixed onto the surface of FRGO, as the cavities on FRGO also had the capacity in bonding metal ions. On the other hand, in the basic condition, hydroxyl group from the base would coordinate with palladium instead of chloride to lead to the coordination state of palladium changed.18,33 The supposed mechanism was showed in Fig. 2. In the catalytic cycle, the addition of aryl halide onto the palladium coordination centres formed a tetra-coordinated intermediate. Transmetallation between palladium intermediate and the alkyl borate complex followed by the elimination formed the C–C sigma bond. The Pd–FRGO catalyst regenerated, with hydroxyl replaced chloride to coordinate with palladium.
To study the actual function of the catalyst, several reactions were carried out. The in situ prepared catalyst was separated from the solvent with the dissociative PdCl2 removed. After redispersed in DMF, the catalyst was used to the reactions of compound 2 with 7a–f. The results had no different manifestations with the previous reactions, which certified that the Pd(II)–FRGO played a main role. Tolerance of air and water of this catalyst was also tested. Reactions of compound 2 with 7a–f were carried out in atmosphere successfully. The turnover frequency of this reaction can be up to 9700.
The palladium-catalysed coupling of olefins with aryl or vinyl halides, known as the Heck reaction, is a standard method for carbon–carbon bond formation in organic synthesis.4,36–38 Our in situ prepared catalyst not only showed high efficiency in Suzuki reaction, but also good performance in Heck reaction. The reaction of iodobenzene (15) and butyl acrylate (18) was carried out with Pd(II)–FRGO, triethylamine and tetrabutylammoniumacetate (TBAA) in DMF at 120 °C.16 The coupling proceeded smoothly to give butyl cinnamate with 84% yield. Moreover, aryl bromides (2–5, 16, 17) were also suitable substrates for the coupling with 62–89% yields. The coupling of aryl halides (2–4, 15–17) and styrene (19) also proceeded smoothly to give the corresponding stilbenes with 66–82% yields (Table 2).
| Entry | A | B | Yield (%) |
|---|---|---|---|
| a Structure of the products see in the ESI.b Butyl acrylate reacted with halides for 20 h at 120 °C while styrene reacted with halides for 10 h at 100 °C. | |||
| 1 | 2 | 18 | 20a, 84 |
| 2 | 3 | 18 | 20b, 64 |
| 3 | 4 | 18 | 20c, 83 |
| 4 | 5 | 18 | 20d, 62 |
| 5 | 15 | 18 | 20e, 89 |
| 6 | 16 | 18 | 20f, 86 |
| 7 | 17 | 18 | 20g, 87 |
| 8 | 2 | 19 | 21a, 71 |
| 9 | 3 | 19 | 21b, 66 |
| 10 | 4 | 19 | 21c, 82 |
| 11 | 15 | 19 | 21d, 82 |
| 12 | 16 | 19 | 21e, 72 |
| 13 | 17 | 19 | 21f, 79 |
The in situ prepared catalyst was also applied to a variety of other organic transformations. The development of C–H bond functionalization reactions (C–H bond activation) is an important topic.39 In the past decade, palladium-catalyzed C–H activation/C–C bond-forming reactions have emerged as promising new catalytic transformations.17,40–42 We applied Pd(II)–FRGO for the C–H bond functionalization reactions of thiophenes.43,44 These reactions of aryl bromide (3, 4, 16, 17) with 2-ethylthiophene (22) were carried out with Pd(II)–FRGO (0.5 mol%) and CsOAc in DMF to give products (23a–23d) with 67–85% yields (Table 3).
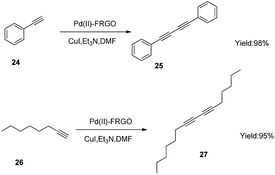
Another interesting phenomenon was discovered when the catalyst was applied to Sonogashira reaction. Phenyl acetylene (24), aryl bromide, Pd(II)–FRGO, cuprous iodide, base and DMF were treated under nitrogen. The product was the Glaser coupling reaction product 1,4-diphenylbuta-1,3-diyne (25), while aryl bromide did not take part in the reaction. When we used 1-octyne (26) instead of phenyl acetylene, hexadeca-7,9-diyne (27) was the only product (Scheme 1). This high efficiency catalyst in Glaser reaction may be applied into new kind of poly-alkyne materials or be used as a new click reaction strategy in macro cycle synthesis or modification of polymer chains.45–48
Recently, a similar work about this reaction was described by Lei's group.49 They reported an efficient alkyne C–H activation and homocoupling procedure, which indicated that a Cu(II)/Cu(I) synergistic cooperation might be involved. In our work, Pd(II)–FRGO was used to instead of Cu(II). To confirm the hypothesis, the control experiments were carried out in the same way as Pd(II)–FRGO except for the only use of CuI and PdCl2. The reaction did not occurred, proving that Cu(I) and Pd(II)–FRGO had the synergetic effect in this C–H activation process.
Conclusions
In summary, a new designed Pd(II)–FRGO complex shows favourable catalytic property in several kinds of coupling reactions. Existence form of palladium as a Pd(II) salt brings many benefits, especially, low cost, simple material, excellent storage performance and high stability. This catalyst can be widely used in Suzuki–Miyaura reaction and Glaser coupling reaction, in all the examples we have tried, the reactions are carried out mildly at room temperature with a low catalyst amount and a high yield. In addition, the reaction is largely unaffected in the presence of air. Meanwhile, Mizoroki–Heck reaction and C–H bond functionalization reactions of thiophenes can also be catalysed. By this means, a new methodology in palladium catalysed coupling reaction is developed. Two stable and commercial materials in the same solvent formed an effective catalyst immediately after mixing, which can be applied in C–C coupling reactions without any sample pre-treatment. Low cost, perfect stability and convenience in use makes this novel catalyst to be a promising catalyst in future.Notes and references
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133–173 CrossRef CAS PubMed.
- T. Y. Luh, M. K. Leung and K. T. Wong, Chem. Rev., 2000, 100, 3187–3204 CrossRef CAS PubMed.
- A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945–2963 CrossRef CAS PubMed.
- F. Diederich and P. J. Stang, Metal-catalyzed cross-coupling reactions, John Wiley & Sons, 2008 Search PubMed.
- N. Miyaura and S. L. Buchwald, Cross-coupling reactions: a practical guide, Springer Science & Business Media, 2002 Search PubMed.
- J. P. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651–2710 CrossRef CAS PubMed.
- K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442–4489 CrossRef CAS PubMed.
- N. T. S. Phan, M. Van Der Sluys and C. W. Jones, Adv. Synth. Catal., 2006, 348, 609–679 CrossRef CAS PubMed.
- A. Balanta, C. Godard and C. Claver, Chem. Soc. Rev., 2011, 40, 4973–4985 RSC.
- L. Djakovitch, K. Köhler and J. G. Vries, Nanopart. Catal., 2008, 303–348 CAS.
- J. A. Widegren and R. G. Finke, J. Mol. Catal. A: Chem., 2003, 198, 317–341 CrossRef CAS.
- G. M. Scheuermann, L. Rumi, P. Steurer, W. Bannwarth and R. Mülhaupt, J. Am. Chem. Soc., 2009, 131, 8262–8270 CrossRef CAS PubMed.
- A. Corma, D. Das, H. García and A. Leyva, J. Catal., 2005, 229, 322–331 CrossRef CAS PubMed.
- D. Astruc, Inorg. Chem., 2007, 46, 1884–1894 CrossRef CAS PubMed.
- Y. M. Yamada, Y. Yuyama, T. Sato, S. Fujikawa and Y. Uozumi, Angew. Chem., Int. Ed., 2014, 53, 131–135 CrossRef PubMed.
- X. Chen, K. M. Engle, D. H. Wang and J. Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094–5115 CrossRef CAS PubMed.
- N. Marion and S. P. Nolan, Acc. Chem. Res., 2008, 41, 1440–1449 CrossRef CAS PubMed.
- E. A. Kantchev, C. J. O'Brien and M. G. Organ, Angew. Chem., Int. Ed., 2007, 46, 2768–2813 CrossRef CAS PubMed.
- R. Narayanan and M. A. El-Sayed, J. Am. Chem. Soc., 2003, 125, 8340–8347 CrossRef CAS PubMed.
- B. J. Gallon, R. W. Kojima, R. B. Kaner and P. L. Diaconescu, Angew. Chem., Int. Ed., 2007, 46, 7251–7254 CrossRef CAS PubMed.
- S. Niembro, A. Shafir, A. Vallribera and R. Alibés, Org. Lett., 2008, 10, 3215–3218 CrossRef CAS PubMed.
- C. Putta, V. Sharavath, S. Sarkara and S. Ghosh, RSC Adv., 2015, 5, 6652–6660 RSC.
- K. Mennecke, R. Cecilia, T. N. Glasnov, S. Gruhl, C. Vogt, A. Feldhoff, M. Vargas, C. O. Kappe, U. Kunz and A. Kirschning, Adv. Synth. Catal., 2008, 350, 717–730 CrossRef CAS PubMed.
- T. Tagata and M. Nishida, J. Org. Chem., 2003, 68, 9412–9415 CrossRef CAS PubMed.
- R. B. Bedford, U. G. Singh, R. I. Walton, R. T. Williams and S. A. Davis, Chem. Mater., 2005, 17, 701–706 CrossRef CAS.
- L. Djakovitch and K. Koehler, J. Am. Chem. Soc., 2001, 123, 5990–5999 CrossRef CAS PubMed.
- L. Ren, F. Yang, Y. Li, T. Liu, L. Zhang, G. Ning, Z. Liu, J. Gao and C. Xu, RSC Adv., 2014, 4, 26804–26809 RSC.
- L. Ren, F. Yang, C. Wang, Y. Li, H. Liu, Z. Tu, L. Zhang, Z. Liu, J. Gao and C. Xu, RSC Adv., 2014, 4, 63048–63054 RSC.
- A. Lerf, H. He, M. Forster and J. Klinowski, J. Phys. Chem. B, 1998, 102, 4477–4482 CrossRef CAS.
- D. W. Boukhvalov and M. I. Katsnelson, J. Am. Chem. Soc., 2008, 130, 10697–10701 CrossRef CAS PubMed.
- W. N. Wang, J. J. Gu, W. Hua, X. D. Jia and K. Xi, Chem. Commun., 2014, 50, 8889–8891 RSC.
- Q. S. Zhao, Y. Zhu, Z. Sun, Y. Li, G. Zhang, F. Zhang and X. Fan, J. Mater. Chem. A., 2015, 3, 2609–2616 CAS.
- R. Rossi, F. Bellina and A. Carpita, Synthesis, 2004, 2419–2440 CrossRef PubMed.
- P. Lloyd-Williams and E. Giralt, Chem. Soc. Rev., 2001, 30, 145–157 RSC.
- I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009–3066 CrossRef CAS PubMed.
- L. E. Overman and D. J. Poon, Angew. Chem., Int. Ed., 1997, 36, 518–521 CrossRef CAS PubMed.
- A. Ashimori and L. E. Overman, J. Org. Chem., 1992, 57, 4571–4572 CrossRef CAS.
- R. G. Bergman, Nature, 2007, 446, 391–393 CrossRef CAS PubMed.
- D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174–238 CrossRef CAS PubMed.
- I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173–1193 RSC.
- M. Miura, T. Tsuda, T. Satoh, S. Pivsa-Art and M. Nomura, J. Org. Chem., 1998, 63, 5211–5215 CrossRef CAS.
- L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792–9826 CrossRef CAS PubMed.
- Y. Huang, Z. Lin and R. Cao, Chem.–Eur. J., 2011, 17, 12706–12712 CrossRef CAS PubMed.
- C. J. Duxbury, D. Cummins and A. Heise, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3795–3802 CrossRef CAS PubMed.
- D. Maag, T. Kottke, M. Schulte and A. Godt, J. Org. Chem., 2009, 74, 7733–7742 CrossRef CAS PubMed.
- H. Y. Gao, H. Wagner, D. Zhong, J. H. Franke, A. Studer and H. Fuchs, Angew. Chem., Int. Ed., 2013, 52, 4024–4028 CrossRef CAS PubMed.
- E. Merkul, D. Urselmann and T. J. Müller, Eur. J. Org. Chem., 2011, 2011, 238–242 CrossRef PubMed.
- R. P. Bai, G. H. Zhang, H. Yi, Z. L. Huang, X. T. Qi, C. Liu, J. T. Miller, A. J. Kropf, E. E. Bunel, Y. Lan and A. W. Lei, J. Am. Chem. Soc., 2014, 136, 16760–16763 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supporting data. See DOI: 10.1039/c5ra10585d |
| This journal is © The Royal Society of Chemistry 2015 |