Synthesis and properties of transparent polyimides derived from trans-1,4-bis(2,3-dicarboxyphenoxy)cyclohexane dianhydride†
Yu Zhouab,
Guofei Chen*a,
Huiwen Zhaoa,
Liping Songb and
Xingzhong Fang*a
aNingbo Key Laboratory of Polymer Materials, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo, Zhejiang 315201, People’s Republic of China. E-mail: gfchen@nimte.ac.cn; fxzhong@nimte.ac.cn
bDepartment of Chemistry, School of Science, Shanghai University, Shanghai, 200444, People’s Republic of China
First published on 5th June 2015
Abstract
A series of transparent polyimides was prepared from trans-1,4-bis(2,3-dicarboxyphenoxy)cyclohexane dianhydride (trans-3,3′-CHDPA) with various aromatic diamines via one-step solution polycondensation. The glass transition temperatures (Tgs) of the resulting polyimides were in the range of 206–255 °C. These polyimide films showed high optical transparency with cut-off wavelengths of 370–379 nm, and they exhibited good mechanical properties with tensile strengths of 65–88 MPa, tensile moduli of 1.7–2.4 GPa, and elongations at break of 4.7–7.5%. Compared with polyimides based on trans-1,4-bis(3,4-dicarboxyphenoxy)cyclohexane dianhydride (trans-4,4′-CHDPA), the polyimides derived from trans-3,3′-CHDPA showed higher Tg, better solubility and optical transparency due to the 3- and 4-position isomeric effect.
1. Introduction
Colorless high-temperature polymeric materials are one of the most attractive research interests due to the great requirement in some special optical fields, such as flexible display substrates, nonlinear optical (NLO) waveguide materials and optical half-wave plates.1,2 Polyimides (PIs) have been deemed to be a potential candidate owing to their excellent thermal and mechanical stabilities, and are widely used in the microelectronics industry.3–6 However, the widespread applications of polyimides in optical fields are often limited because of the deep coloration which is caused by the strong intra- and intermolecular charge transfer (CT) interactions.7,8 Therefore, a lot of effort has been made to decrease the coloration based on structural design by modification of dianhydrides and diamines, including the incorporation of trifluoromethyl groups,9–21 bulky groups,22–24 unsymmetrical20,25,26 and alicyclic units27–38 into the polymer chains. Our group has also reported transparent semi-aromatic polyimides by the incorporation of aliphatic cyclohexane-1,4-diol into two phthalic anhydrides or diamines, which introduced an alicyclic structure without sacrificing the reactivity of the monomers.39,40On the other hand, compared with 4,4′-position polyimides, isomeric polyimides derived from 3,4′-dianhydrides or 3,3′-dianhydrides have become a new research interest for researchers due to their own outstanding characteristics, such as higher glass transition temperature (Tg), better solubility, lower melt viscosity, etc.41 However, most of the studies focused on the isomeric dianhydrides and/or diamines at different substituted positions (such as the 3,3′-substituted position and 3,4′-substituted position), and paid less attention to stereoisomers, for example, the trans and cis configuration. Toshihiko Matsumoto et al. first reported stereoisomeric polyimides derived from trans- and cis-tetracarboxylic dianhydride bearing a cycloaliphatic structure, and indicated that the trans-polyimides had better transparency and solubility but lower Tg than the cis-polyimides.31 In addition, trans-polyimides derived from trans-1,2,4,5-cyclohexanetetracarboxylic dianhydride showed higher transparency, better solubility but lower Tg than cis-polyimides, which was reported by Masatoshi Hasegawa.37 Stereoisomeric polyimides derived from cis- and trans-1,2,3,4-cyclohexanetetracarboxylic dianhydrides have also been synthesized and showed that trans-polyimides had lower Tg and lower solubility than the corresponding cis-polyimides.38 It was found that the trans-polyimides which were bearing a cycloaliphatic structure had good transparency but lower Tgs. Recently, our group reported the trans and cis configuration isomeric effect of polyimides derived from trans- and cis-1,4-bis(3,4-dicarboxyphenoxy)cyclohexane (4,4′-CHDPA), as shown in Fig. 1, and found that trans-polymers had higher Tg, better transparency, better chemical solvent resistance and better mechanical properties, which was quite different from the above reports about the trans- and cis isomeric effect on stereoisomeric polyimides.40 Considering isomeric polyimides derived from 3,3′-substituted dianhydrides have higher Tg, better solubility and transparency,41 a new dianhydride monomer containing a 1,4-cyclohexane moiety at the 3-substituted position of phthalic anhydride, trans-1,4-bis(2,3-dicarboxyphenoxy)cyclohexane dianhydride (trans-3,3′-CHDPA), was designed and synthesized in order to enhance the Tg and transparency of the resulting polyimides. The solubility, thermal properties, mechanical properties and optical properties of these polyimides were investigated thoroughly.
2. Experimental
2.1. Materials
Cyclohexane-1,4-diol (cis + trans, 98%, Jingtan Qingcheng Environmental Technologies Co., Ltd.), 3-nitrophthalonitrile (98%, Leadership Chemical Technologies Co., Ltd.) and sodium hydride (60%, Sinopharm Chemical Reagent Co., Ltd.) were used as received. 2,2′-Bis(trifluoromethyl)biphenyl-4,4′-diamine (TFDB, 98%, Changzhou Sunlight Pharmaceutical Co., Ltd.) and 4,4′-diaminodiphenyl ether (ODA, 98%, Aladdin) were purified by vacuum sublimation prior to use. Bis(4-amino-2-trifluoromethylphenyl) ether (TFODA)42 and bis[4-(4-amino-2-trifluoromethylphenoxy)phenyl] sulfone (6F-BAPS)43 were synthesized in our laboratory according to the literature. Bis[4-(3-aminodiphenoxy)phenyl] sulfone (m-BAPS, 98%), 4,4′-(1,3-phenylenedioxy)dianiline (TPER, 98%) and 4,4′-(9H-fluorene-9,9-diyl)dianiline (DFA, 98%) were purchased from Changzhou Sunlight Pharmaceutical Co., Ltd. and recrystallized from ethanol before use. N,N-Dimethylformamide (DMF) and N,N-dimethylacetamide (DMAc) were distilled from calcium hydride and stored over 4 Å molecular sieves. All other reagents for the study were commercially obtained and used as received without further purification.2.2. Instrumentation
Fourier transform infrared (FT-IR) spectra of the powder samples were recorded with a Thermo Nicolet 6700 FT-IR spectrometer. All of the FT-IR spectra of the polyimide film samples were collected in the attenuated total reflection (ATR) mode with a 4 cm−1 resolution for 128 scans each by using a Cary 640 spectrometer (Agilent, Australia). Nuclear magnetic resonance (NMR) spectra were performed on a Bruker 400 AVANCE III spectrometer operating at 400 MHz for 1H NMR and 100 MHz for 13C NMR using dimethyl sulfoxide-d6 (DMSO-d6) as the solvent. The inherent viscosities of the polymers were measured at 30 ± 0.1 °C with an Ubbelohde viscometer and the concentration was 0.5 g dL−1 in m-cresol or DMAc. The weight-average molecular weights (Mw) and number-average molecular weights (Mn) were obtained via gel permeation chromatography (GPC) on the basis of polystyrene calibration on a PL-GPC 220 instrument with CHCl3 as an eluent at a flow rate of 1.0 mL min−1. Differential scanning calorimetry (DSC) measurements of the polyimides were performed on a Mettler Toledo-DSC I at a heating rate of 20 °C min−1 under nitrogen atmosphere, and the temperature at the middle of the thermal transition from the second heating scan was assigned as the glass transition temperature (Tg). The melting point (m.p.) of the synthesized monomers was measured by micro melting point apparatus. Thermo gravimetric analyses (TGA) of the polyimides were performed on a Mettler Toledo-TGA/DSC I instrument to evaluate the thermal stability of the polyimides at a heating rate of 10 °C min−1 from 50 °C to 800 °C under nitrogen or air atmosphere (flow rate of 50 mL min−1). The mechanical properties of the polyimide films such as tensile modulus, tensile strength, and elongation at break were measured and averaged on at least six film specimens by an Instron model 5567 tensile tester at room temperature. Ultraviolet-visible (UV-vis) spectra of the polymer films were recorded on a Lambda 950 UV/Vis/NIR Spectrophotometer. The wide-angle X-ray diffraction (WAXD) measurement of the polyimide films was undertaken on a Bruker D8 Advance with Cu Kα radiation (40 kV, 40 mA) at a scanning rate of 5° min−1 from 5 to 50°.2.3. Monomer synthesis
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching). 1H NMR δH (400 MHz, DMSO-d6): 7.89–7.82 (2H, m, Hb), 7.77 (2H, t, Ha), 7.66 (2H, d, Hc), 4.96 (1.3H, s, Hd), 4.85 (0.7H, s, Hd′), 2.08–2.01 (2H, m, He), 1.95–1.88 (4H, m, He′, Hf′) and 1.81–1.72 (2H, m, Hf).
N stretching). 1H NMR δH (400 MHz, DMSO-d6): 7.89–7.82 (2H, m, Hb), 7.77 (2H, t, Ha), 7.66 (2H, d, Hc), 4.96 (1.3H, s, Hd), 4.85 (0.7H, s, Hd′), 2.08–2.01 (2H, m, He), 1.95–1.88 (4H, m, He′, Hf′) and 1.81–1.72 (2H, m, Hf).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1291, 1259, 1068, 1019 cm−1 (C–O stretching). 1H NMR δH (400 MHz; DMSO-d6): 12.93 (4H, s, Hg), 7.48–7.37 (6H, m, Ha, Hb, Hc), 4.65 (1.9H, s, Hd), 4.50 (0.1H, s, Hd′), 2.02–1.92 (4H, m, He), 1.92–1.67 (0.3H, m, He′, Hf′) and 1.67–1.57 (4H, m, Hf). 13C NMR δC (100 MHz, DMSO-d6, ppm): 168.1, 167.0, 153.8, 130.1, 129.5, 128.1, 121.7, 118.8, 73.5, 25.9.
O stretching), 1291, 1259, 1068, 1019 cm−1 (C–O stretching). 1H NMR δH (400 MHz; DMSO-d6): 12.93 (4H, s, Hg), 7.48–7.37 (6H, m, Ha, Hb, Hc), 4.65 (1.9H, s, Hd), 4.50 (0.1H, s, Hd′), 2.02–1.92 (4H, m, He), 1.92–1.67 (0.3H, m, He′, Hf′) and 1.67–1.57 (4H, m, Hf). 13C NMR δC (100 MHz, DMSO-d6, ppm): 168.1, 167.0, 153.8, 130.1, 129.5, 128.1, 121.7, 118.8, 73.5, 25.9.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1763 cm−1 (sym C
O stretching), 1763 cm−1 (sym C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1287, 1262, 1052, 1009 cm−1 (C–O stretching).
O stretching), 1287, 1262, 1052, 1009 cm−1 (C–O stretching).The synthesis of polyimide (PI-2) is described below as an example to illustrate the general synthetic route for the preparation: a mixture of trans-3,3′-CHDPA (1.6663 g, 4.0 mmol), diamine TFODA (1.3451 g, 4.0 mmol), m-cresol (15.0 mL) and 3 drops of isoquinoline in a 50 mL flask was stirred in nitrogen atmosphere at 195 °C for 8 h. The reaction mixture was diluted with m-cresol and slowly poured into ethanol which was being vigorously stirred. The precipitate was collected by filtration, washed with hot ethanol and then dried.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1720 cm−1 (sym C
O stretching), 1720 cm−1 (sym C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1379 cm−1 (C–N stretching). 1H NMR δH (400 MHz, DMSO-d6): 8.02 (2H, s, Hg), 7.88–7.78 (4H, m, Hb, Hh), 7.66 (2H, d, Ha), 7.52 (2H, d, Hc), 7.39 (2H, d, Hi), 4.99 (2H, s, Hd), 2.20–2.07 (4H, m, He) and 1.83–1.73 (4H, m, Hf).
O stretching), 1379 cm−1 (C–N stretching). 1H NMR δH (400 MHz, DMSO-d6): 8.02 (2H, s, Hg), 7.88–7.78 (4H, m, Hb, Hh), 7.66 (2H, d, Ha), 7.52 (2H, d, Hc), 7.39 (2H, d, Hi), 4.99 (2H, s, Hd), 2.20–2.07 (4H, m, He) and 1.83–1.73 (4H, m, Hf).
PI-1. FT-IR (film): 1779 cm−1 (asym C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1721 cm−1 (sym C
O stretching), 1721 cm−1 (sym C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1370 cm−1 (C–N stretching).
O stretching), 1370 cm−1 (C–N stretching).
PI-3. FT-IR (film): 1777 cm−1 (asym C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1720 cm−1 (sym C
O stretching), 1720 cm−1 (sym C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1380 cm−1 (C–N stretching).
O stretching), 1380 cm−1 (C–N stretching).
PI-4. FT-IR (film): 1775 cm−1 (asym C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1714 cm−1 (sym C
O stretching), 1714 cm−1 (sym C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1376 cm−1 (C–N stretching).
O stretching), 1376 cm−1 (C–N stretching).
PI-5. FT-IR (film): 1779 cm−1 (asym C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1722 cm−1 (sym C
O stretching), 1722 cm−1 (sym C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1360 cm−1 (C–N stretching).
O stretching), 1360 cm−1 (C–N stretching).
PI-6. FT-IR (film): 1775 cm−1 (asym C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1712 cm−1 (sym C
O stretching), 1712 cm−1 (sym C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1379 cm−1 (C–N stretching).
O stretching), 1379 cm−1 (C–N stretching).
PI-7. FT-IR (film): 1775 cm−1 (asym C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1713 cm−1 (sym C
O stretching), 1713 cm−1 (sym C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1378 cm−1 (C–N stretching).
O stretching), 1378 cm−1 (C–N stretching).
3. Results and discussion
3.1. Monomer synthesis
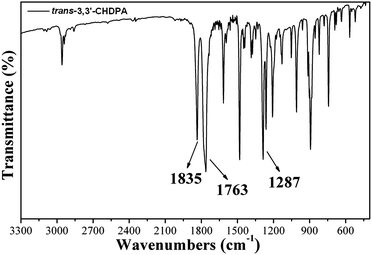
Bis(ether dinitrile) was prepared through the nucleophilic substitution reaction of cyclohexane-1,4-diol with 3-nitrophthalonitrile in the presence of sodium hydride (NaH) in anhydrous N,N-dimethylformamide (DMF) (Scheme 1). The mole ratio of the trans/cis isomers in the 1H NMR spectrum of bis(ether dinitrile) was 1.8/1 and was calculated using the integral ratio of the corresponding peak areas (Hd) to (Hd′) (Fig. 2). 1,4-Bis(2,3-dicarboxyphenoxy)cyclohexane was formed by the hydrolysis of the corresponding bis(ether dinitrile), and the mole ratio of the trans/cis isomers was changed to 19/1 according to the integral ratio of the corresponding peak areas (Hd) to (Hd′) of tetraacid in the 1H NMR spectrum (Fig. 3). It should be noted that the cis configuration had been converted to the trans configuration during the hydrolysis procedure in harsh reaction conditions and this may have been because of the more thermodynamic stable trans configuration. The bis(ether anhydride) monomer, trans-3,3′-CHDPA was afforded by the dehydration of the resulting tetraacid using acetic anhydride. In the IR spectrum of the bis(ether anhydride), the strong absorption bands around 1835 cm−1 (vasymC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 1763 cm−1 (vsymC
O) and 1763 cm−1 (vsymC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) were assigned to the phthalic anhydride (Fig. 4). The observed bands in the region of 1000–1300 cm−1 were due to C–O stretching. The trans configuration of 3,3′-CHDPA was deduced from the 1H NMR spectrum of the corresponding polyimide PI-2 although recording the 1H NMR spectrum of trans-3,3′-CHDPA was not possible due to poor solubility in deuterated solvents such as CDCl3, DMSO-d6, DMF-d7.
O) were assigned to the phthalic anhydride (Fig. 4). The observed bands in the region of 1000–1300 cm−1 were due to C–O stretching. The trans configuration of 3,3′-CHDPA was deduced from the 1H NMR spectrum of the corresponding polyimide PI-2 although recording the 1H NMR spectrum of trans-3,3′-CHDPA was not possible due to poor solubility in deuterated solvents such as CDCl3, DMSO-d6, DMF-d7.
3.2. Synthesis of polyimides
The polyimides were synthesized from the diahydride monomer trans-3,3′-CHDPA and various aromatic diamines (TFDB, TFODA, 6F-BAPS, m-BAPS, DFA, ODA, and TPER) in m-cresol via a conventional one-step method (Scheme 2). The inherent viscosities of the prepared polyimides PI-2 to PI-7 were between 0.34–0.96 dL g−1 in m-cresol at 30 °C and the inherent viscosity of PI-1 was 1.23 dL g−1 in DMAc due to its poor solubility in m-cresol (Table 1). The molecular weights of these PIs were determined by GPC in CHCl3 relative to polystyrene standards and they were in the range of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800–158
800–158![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 for Mw and 41
900 for Mw and 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200–62
200–62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 for Mn with Mw/Mn values of 1.84–2.55 except for the polyimides PI-1, PI-6 and PI-7. The chemical structure of the polyimides was confirmed by 1H NMR and FT-IR spectroscopy. The representative 1H NMR spectrum of PI-2 is shown in Fig. 5. All of the signals have been assigned to the protons of the repeating unit, and completed imidization was confirmed by the absence of the corresponding amide signals and carboxylic acid functions. The signals in the region of 4.99, 2.20–2.00 and 1.90–1.73 ppm were assigned to the methine proton (Hd), equatorial methylene protons (He) and axial methylene protons (Hf) of the cyclohexane moiety with a chair form in the polyimides, respectively. Furthermore, the COSY spectrum of PI-2 (Fig. S1†) was measured for further assignment of the aromatic protons. It should be noted that the configuration of polyimide PI-2 was confirmed to be trans due to the absence of signals corresponding to cis configuration in the 1H NMR spectrum, which also proved that the configuration of 3,3′-CHDPA was trans. The typical IR spectrum of PI-2 is shown in Fig. 6. The strong absorption bands around 1777 cm−1 (vasymC
400 for Mn with Mw/Mn values of 1.84–2.55 except for the polyimides PI-1, PI-6 and PI-7. The chemical structure of the polyimides was confirmed by 1H NMR and FT-IR spectroscopy. The representative 1H NMR spectrum of PI-2 is shown in Fig. 5. All of the signals have been assigned to the protons of the repeating unit, and completed imidization was confirmed by the absence of the corresponding amide signals and carboxylic acid functions. The signals in the region of 4.99, 2.20–2.00 and 1.90–1.73 ppm were assigned to the methine proton (Hd), equatorial methylene protons (He) and axial methylene protons (Hf) of the cyclohexane moiety with a chair form in the polyimides, respectively. Furthermore, the COSY spectrum of PI-2 (Fig. S1†) was measured for further assignment of the aromatic protons. It should be noted that the configuration of polyimide PI-2 was confirmed to be trans due to the absence of signals corresponding to cis configuration in the 1H NMR spectrum, which also proved that the configuration of 3,3′-CHDPA was trans. The typical IR spectrum of PI-2 is shown in Fig. 6. The strong absorption bands around 1777 cm−1 (vasymC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1720 cm−1 (vsymC
O), 1720 cm−1 (vsymC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and 1379 cm−1 (vC–N imide) were assigned to the phthalimide unit. The observed bands in the region of 1000–1300 cm−1 were due to C–O and C–F stretching vibrations.
O), and 1379 cm−1 (vC–N imide) were assigned to the phthalimide unit. The observed bands in the region of 1000–1300 cm−1 were due to C–O and C–F stretching vibrations.
| PI | ηinha (dL g−1) | Mw (g mol−1) | Mn (g mol−1) | Mw/Mn | Tgb (°C) | Td5%c (°C) | Td10%c (°C) | ||
|---|---|---|---|---|---|---|---|---|---|
| In N2 | In air | In N2 | In air | ||||||
| a The inherent viscosities of polyimides were measured at a concentration of 0.5 g dL−1 in m-cresol at 30 °C.b Baseline shift in the second heating DSC traces, with a heating rate of 20 °C min−1.c Temperatures at which 5% weight loss and 10% weight loss were recorded by TGA at a heating rate of 10 °C min−1.d The inherent viscosity was measured in DMAc due to the poor solubility of PI-1 in m-cresol. | |||||||||
| PI-1 | 1.23d | — | — | — | 255 | 368 | 362 | 375 | 372 |
| PI-2 | 0.96 | 158![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
2.55 | 245 | 365 | 368 | 374 | 374 |
| PI-3 | 0.68 | 135![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
2.48 | 232 | 370 | 370 | 390 | 396 |
| PI-4 | 0.34 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.84 | 208 | 368 | 376 | 389 | 415 |
| PI-5 | 0.42 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
2.08 | 225 | 369 | 375 | 384 | 393 |
| PI-6 | 0.47 | — | — | — | 240 | 367 | 372 | 379 | 381 |
| PI-7 | 0.47 | — | — | — | 206 | 374 | 376 | 387 | 388 |
3.3. Thermal properties
The thermal behavior data of the polyimides are listed in Table 1. The Tgs of the polyimides were in the range of 206–255 °C. Generally, Tg is correlated with the stiffness and conformation of the polymer chain. The highest Tg value was for PI-1 and it was attributed to the rigidity of the TFDB moiety while there were lower Tg values for PI-4 and PI-7 and this may have been due to the presence of two flexible ether linkages and the meta substituent of the m-BAPS and TPER moieties. In addition, PI-2 containing a trifluoromethyl group in the side chain had a higher Tg than the corresponding polyimide PI-6 without the trifluoromethyl group. The Tg of PI-5 was higher than PI-3 and PI-4 due to the bulky tetraphenylene moiety in the diamine which would restrain the rotation of the polymer chain. The Tgs of PI-2 and PI-4, based on trans-3,3′-CHDPA, were higher than the corresponding polyimides derived from trans-1,4-bis(3,4-dicarboxyphenoxy)cyclohexane (trans-4,4′-CHDPA), except for PI-1 with TFDB as the diamine.40 Such behavior may have been observed because of the steric effect of the suppressed rotation of the ether bond between the 3-substituted phthalimide and the 1,4-cyclohexane.41The thermal stability of the polyimides was evaluated by dynamic TGA conducted at a heating rate of 10 °C min−1. The temperature of 5% weight loss (Td5%) in nitrogen and air atmospheres was determined from original TGA thermograms, which are listed in the Table 1 and the typical TGA curves of PI-3 and PI-4 are shown in Fig. 7. The Td5% values of these PIs were recorded in the range of 365–374 °C in nitrogen and 362–376 °C in air. The low decomposition temperature in comparison with fully aromatic polyimides was caused by the first decomposition of the aliphatic cyclohexane moiety at elevated temperature.13,44 However, the polyimides synthesized from trans-3,3′-CHDPA showed a lower Td than the PIs derived from trans- and cis-4,4′-CHDPA due to the 1,4-cyclohexane moiety in the polymers which would decrease the mobility of polymer and make the polymers absorb less thermal energy.45
3.4. Solubility
The solubility of the polyimides was tested qualitatively in various organic solvents, and the results are shown in Table 2. The solubility of PI-2 and PI-3 is better than PI-6 and PI-4, owing to the CF3 group which inhibited close packing and reduced the inter-chain interaction which enhanced the solubility. PI-5 also showed better solubility in common organic solvents because of the bulky cardo group. In addition, PI-6 and PI-7 showed comparatively poor solubility compared to the other PIs due to the regularity of their polymer chains and the increasing denser chain stacking, thereby making it difficult for the solvent to attack. It was also observed that the polyimides derived from trans-3,3′-CHDPA showed better solubility than the trans-4,4′-CHDPA based polyimides,40 probably because the trans-3,3′-CHDPA moiety in the polymer chain disturbed the regularity of the molecular chain, which reduced the intermolecular interactions and hindered the close stacking of the molecular chains, and therefore the solvent molecules could penetrate easily.41| PI | Solventsb | ||||||
|---|---|---|---|---|---|---|---|
| m-cresol | DMAc | DMF | NMP | DMSO | CHCl3 | THF | |
| a The qualitative solubility was tested with 10 mg samples in 1 mL of solvent. ++: Soluble at room temperature; +: soluble on heating; +−: partially soluble on heating.b DMAc: N,N-dimethylacetamide; DMF: N,N-dimethylformamide; NMP: N-methyl-2-pyrrolidone; DMSO: dimethyl sulfoxide; THF: tetrahydrofuran. | |||||||
| PI-1 | + | ++ | +− | ++ | +− | +− | +− |
| PI-2 | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| PI-3 | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| PI-4 | ++ | ++ | ++ | ++ | ++ | ++ | +− |
| PI-5 | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| PI-6 | ++ | +− | +− | ++ | +− | ++ | +− |
| PI-7 | ++ | +− | +− | ++ | +− | +− | +− |
3.5. Mechanical properties
The mechanical properties of the flexible polyimide films are listed in Table 3. The polyimide films exhibited good mechanical properties with tensile strengths of 65–88 MPa, tensile moduli of 1.7–2.4 GPa, and elongations at break of 4.7–7.5%. Among these polyimide films, the tensile strength and tensile modulus of PI-1 and PI-5 were more than 85 MPa and 2.3 GPa, respectively, which were attributed to the rigid structure in the polymers’ backbone. Polyimides based on trans-4,4′-CHDPA exhibited higher elongation and somewhat higher tensile strength and modulus than the other isomers.40 For the polyimides based on trans-3,3′-CHDPA, the 3,3′-position may tend to cyclize in some instances and they seldom attain molecular weights as high as the others.41| PI | Tensile strength (MPa) | Tensile modulus (GPa) | Elongation at break (%) |
|---|---|---|---|
| PI-1 | 85.8 | 2.3 | 5.4 |
| PI-2 | 65.1 | 1.7 | 5.5 |
| PI-3 | 79.3 | 2.2 | 5.5 |
| PI-4 | 75.5 | 2.2 | 4.7 |
| PI-5 | 88.3 | 2.4 | 7.0 |
| PI-6 | 74.9 | 2.1 | 5.6 |
| PI-7 | 74.0 | 1.8 | 7.5 |
3.6. X-ray diffraction
The crystallinity of the polyimide films was analyzed by wide-angle X-ray diffraction (WAXD). The WAXD patterns of all the films were broad without obvious peak features, which indicated that they were all amorphous (Fig. S2†). The amorphous nature of the polyimides could be attributed to the introduction of the alicyclic hexane moiety in the dianhydride resulting in loose chain packing and aggregation.363.7. Optical properties
The transmission UV-visible spectra were measured for the thin polyimide films. The UV-visible spectra of some representative polyimide films are illustrated in Fig. 8, and the cut-off wavelength (absorption edge, λ0) and the transmittance at 400, 450, 500 nm from these spectra are listed in Table 4. All of the polyimide films exhibited cut-off wavelengths shorter than 379 nm and were highly transparent. The cyclohexane moiety in the dianhydride weakened both intra- and intermolecular charge transfer interactions by breaking the conjugation and decreasing the electron-acceptability of the dianhydride. Furthermore, compared with PIs derived from trans-4,4′-CHDPA, the PIs synthesized from trans-3,3′-CHDPA showed better transparency due to the 3,4′-position isomeric effect which was effective in decreasing charge transfer (CT) complex formation between the polymer chains through steric hindrance and increasing free volume.12 Moreover, the incorporation of the trifluoromethyl group (PI-1, PI-2, PI-3) and bulky group (PI-5) in the diamines also enhanced the optical transparency by decreasing CT complex formation between the polymer chains through steric hindrance and an inductive effect (decreasing the electron-donating property of the diamine moieties).8 Among all the polyimides, PI-1 showed the best optical transparency.4. Conclusion
A series of polyimides derived from trans-1,4-bis(2,3-dicarboxyphenoxy)cyclohexane dianhydride (trans-3,3′-CHDPA), containing 1,4-cyclohexane at the 3-position of the phthalic anhydride, was synthesized via a one-step method and could form transparent, flexible, and tough films with tensile strengths of 65–88 MPa, tensile moduli of 1.7–2.4 GPa and elongations at break of 4.7–7.5%. The polyimide films from trans-3,3′-CHDPA showed higher Tgs and lower coloration compared with trans-4,4′-CHDPA-based polyimides. This proved the combination of 1,4-cyclohexane and the 3-substituted position on the phthalic anhydride, which could decrease the electron-acceptability of the dianhydride and reduce the intra-/intermolecular interaction, was a successful method for reducing charge transfer complex formation and enhancing the Tg and solubility. PI-1 exhibited the highest Tg, and best optical transparency and so may be a promising film for further application.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (Grant no. 51403225), Zhejiang Provincial Natural Science Foundation of China (Grant no. LQ12B04001), and Guangdong Province and Chinese Academy of Sciences Comprehensive Strategic Cooperation Project (no. 2013B091000003).Notes and references
- G. Maier, Prog. Polym. Sci., 2001, 26, 3–65 CrossRef CAS.
- C. M. Choi, Y. Kim and S. C. Ha, Prog. Polym. Sci., 2008, 33, 581–630 CrossRef PubMed.
- L. K. Mittal, Polyimide: synthesis, characterization, and application, Plenum, New York, 1984 Search PubMed.
- C. Feger, M. M. Khojasteh and J. E. McGrath, Polyimides: chemistry and characterization, Elsevier, Amsterdam, 1994 Search PubMed.
- M. K. Ghosh and K. L. Mittal, Polyimides: fundamentals and applications, Marcel Dekker, New York, 1996 Search PubMed.
- M. X. Ding, Polyimides: chemistry, relationship between structure and properties and materials, Science Press, Beijing, 2006 Search PubMed.
- M. Hasegawa and K. Horie, Prog. Polym. Sci., 2001, 26, 259–335 CrossRef CAS.
- S. Ando, T. Matsuura and S. Sasaki, Polym. J., 1997, 29, 69–76 CrossRef CAS.
- H. Masatoshi, I. Tomohiro, I. Junichi, S. Kentaro and F. Mari, Eur. Polym. J., 2013, 49, 3657–3672 CrossRef PubMed.
- Y. Y. Chen, C. P. Yang and S. H. Hsiao, Eur. Polym. J., 2006, 42, 1705–1715 CrossRef CAS PubMed.
- C. P. Yang, Y. Y. Su and M. Y. Hsu, Polym. J., 2006, 38, 132–144 CrossRef CAS.
- C. P. Yang and Y. Y. Su, Polymer, 2005, 46, 5797–5807 CrossRef CAS PubMed.
- C. P. Yang, Y. Y. Su, S. J. Wen and S. H. Hsiao, Polymer, 2006, 47, 7021–7033 CrossRef CAS PubMed.
- C. P. Yang, J. M. Wang, Y. Y. Su and S. H. Hsiao, Macromol. Chem. Phys., 2006, 207, 1049–1061 CrossRef CAS PubMed.
- C. P. Yang, Y. Y. Su and S. H. Hsiao, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5909–5922 CrossRef CAS PubMed.
- K. A. Watson, F. L. Palmieri and J. W. Connell, Macromolecules, 2002, 35, 4968–4974 CrossRef CAS.
- A. K. St. Clair and T. L. St. Clair, US Pat., 4603061, 1986.
- T. Matsuura, S. Ando, S. Sasaki and F. Yamamoto, Macromolecules, 1994, 27, 6665–6670 CrossRef CAS.
- C. P. Yang, S. H. Hsiao and K. L. Wu, Polymer, 2003, 44, 7067–7078 CrossRef CAS PubMed.
- C. P. Yang, Y. C. Chen, S. H. Hsiao, W. J. Guo and H. M. Wang, J. Polym. Res., 2010, 17, 779–788 CrossRef CAS PubMed.
- C. P. Yang and M. Y. Hsu, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 1270–1284 CrossRef CAS PubMed.
- Z. Y. Ge, L. Fan and S. Y. Yang, Eur. Polym. J., 2008, 44, 1252–1260 CrossRef CAS PubMed.
- Y. Liu, Y. H. Zhang, S. W. Guan, L. Li and Z. H. Jiang, Polymer, 2008, 49, 5439–5445 CrossRef CAS PubMed.
- Z. Li, J. G. Liu, Z. Q. Gao, Z. H. Yin, L. Fan and S. Y. Yang, Eur. Polym. J., 2009, 45, 1139–1148 CrossRef CAS PubMed.
- C. P. Yang and Y. Y. Su, Polymer, 2005, 46, 5778–5788 CrossRef CAS PubMed.
- C. P. Yang and Y. Y. Su, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 3140–3152 CrossRef CAS PubMed.
- M. Yamada, M. Kusama, T. Matsumoto and T. Kurosaki, Macromolecules, 1993, 26, 4961–4963 CrossRef CAS.
- T. Matsumoto and T. Kurosaki, Macromolecules, 1995, 28, 5684–5685 CrossRef CAS.
- T. Matsumoto and T. Kurosaki, Macromolecules, 1997, 30, 993–1000 CrossRef CAS.
- A. Shiotani, H. Shimazaki and M. Matsuo, Macromol. Mater. Eng., 2001, 286, 434–441 CrossRef CAS.
- T. Matsumoto, Macromolecules, 1999, 32, 4933–4939 CrossRef CAS.
- J. G. Liu, M. H. He, H. W. Zhou, Z. G. Qian, F. S. Wang and S. Y. Yang, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 110–119 CrossRef CAS PubMed.
- A. S. Mathews, I. Kim and S. C. Ha, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5254–5270 CrossRef CAS PubMed.
- T. Yamashita, M. Ogawa, H. Koshikawa and Y. Maekawa, J. Photopolym. Sci. Technol., 2007, 20, 739–742 CrossRef CAS.
- M. Hasegawa, K. K. Asamatsu and K. Koseki, Eur. Polym. J., 2012, 48, 483–498 CrossRef CAS PubMed.
- L. Zhai, S. Y. Yang and L. Fan, Polymer, 2012, 53, 3529–3539 CrossRef CAS PubMed.
- H. Masatoshi, H. Daiki, F. Mari, H. Misako, T. Eiichiro, Y. Shinya, I. Atsushi and K. Takashi, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 575–592 CrossRef PubMed.
- X. Z. Fang, Z. H. Yang, S. B. Zhang, L. X. Gao and M. X. Ding, Polymer, 2004, 45, 2539–2549 CrossRef CAS PubMed.
- Y. J. Hou, G. F. Chen, X. L. Pei and X. Z. Fang, J. Polym. Res., 2012, 19, 9955 CrossRef PubMed.
- G. F. Chen, X. L. Pei, J. T. Liu and X. Z. Fang, J. Polym. Res., 2013, 20, 159 CrossRef.
- M. X. Ding, Prog. Polym. Sci., 2007, 32, 623–668 CrossRef CAS PubMed.
- A. Satoh and A. Morikawa, High Perform. Polym., 2010, 22, 412–427 CrossRef CAS PubMed.
- C. P. Yang, Y. Y. Su and K. L. Wu, J. Polym. Res., 2005, 12, 257–269 CrossRef CAS.
- C. L. Chung, C. P. Yang and S. H. Hsiao, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 3092–3102 CrossRef CAS PubMed.
- J. T. Liu, G. F. Chen and X. Z. Fang, Polym. Adv. Technol., 2014, 25, 329–337 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra07676e |
| This journal is © The Royal Society of Chemistry 2015 |