Dual channel selective fluorescent detection of Al3+ and PPi in mixed aqueous media: DFT studies and cell imaging applications†
Abstract
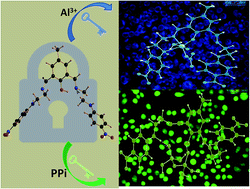
A new, easily synthesizable chemosensor, DFC-EN-p-Ph-NO2, derived by the Schiff base condensation between 2,6-diformyl-p-cresol and N-(4-nitrophenyl)ethylenediamine, with potential N4O donor atoms was found to act as a dual channel (colori- and fluorimetric) sensor towards Al3+ and PPi emitting at 486 nm (blue region) and 534 nm (green region), respectively in MeOH–H2O (8 : 2, v/v) at pH 7.2 (10 mM HEPES buffer), μ = 0.05 M (LiCl), temperature 25 °C. The binding stoichiometries and formation constants of the sensor towards both Al3+ and PPi were determined by the combined UV-Vis and fluorescence titrations and Job's method, and confirmed by MS (m/z) studies. The corresponding detection limits as calculated by the 3σ method are: 7.55 μM and 3.34 μM. The most interesting part of this study is that on addition of 230 μM PPi to an ensemble of DFC-EN-p-Ph-NO2−Al3+ (20 μM Ligand and 380 μM Al3+) the fluorescence is totally quenched but on further addition of PPi a new emission peak appears at 534 nm. All biologically relevant metal ions and toxic heavy metals did not interfere with the detection of Al3+ ion. Its bio-compatibility with respect to its good solubility in mixed organo-aqueous media (MeOH–H2O) and cell permeability with no or negligible cytotoxicity provide good opportunities towards in vitro cell imaging studies of these ions. In particular, the fluorescent detection of PPi was not interfered by the presence of 400 μM of ATP or Pi although most reported PPi sensors that work in aqueous solution displayed cross-sensitivities toward ATP or Pi. The obvious excellent sensing capability of DFC-EN-p-Ph-NO2 towards PPi and Al3+ was further scrutinized in HCT116 cell lines without much cytotoxicity. The modes of 1 : 1 binding of DFC-EN-p-Ph-NO2 towards Al3+ and PPi were delineated by DFT calculations.

 Please wait while we load your content...
Please wait while we load your content...