A direct warm-white-light CaLa2(MoO4)4: Tb3+, Sm3+ phosphor with tunable color tone via energy transfer for white LEDs
Abstract
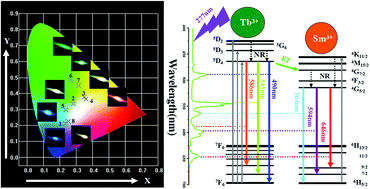
A series of Tb3+, Sm3+ co-doped CaLa2(MoO4)4 phosphors have been prepared via a solvothermal method without further sintering. The CaLa2(MoO4)4:1% Tb3+, x% Sm3+ (x = 0.0–5.0) phosphors were characterized by X-ray diffraction (XRD), field-emission scanning electron microscopy (FE-SEM) and photoluminescence (PL) spectra. Upon 277 nm excitation, the phosphors exhibit strong green emission of the Tb3+ ions and red-orange emission of the Sm3+ ions, the quenching concentration of Sm3+ is determined to be about 4%. The critical distance between Tb3+ and Sm3+ has been calculated to be about 14.3 Å and the energy transfer from Tb3+ to Sm3+ occurs through a dipole–dipole interaction. The color tone of the obtained phosphors is easily modulated from blue to cool-white, green, and ultimately to warm-white-light. Furthermore, the relationship between the value of CCT for warm-white-light and the rare earth ion (Tb3+, Sm3+) concentration was investigated in detail. These results reveal that this kind of phosphor is a potential candidate for white LEDs.

 Please wait while we load your content...
Please wait while we load your content...