Synthesis of conductive magnetic nickel microspheres and their applications in anisotropic conductive film and water treatment
Bing Yu*,
Feng Zhai,
Hailin Cong*,
Dong Wang,
Qiaohong Peng,
Shijing Yang and
Ruixia Yang
Laboratory for New Fiber Materials and Modern Textile, Growing Base for State Key Laboratory, College of Chemical Engineering, Qingdao University, China. E-mail: yubingqdu@yahoo.com; hailincong@yahoo.com; Fax: +86 532 85955529; Tel: +86 532 85953995
First published on 10th September 2015
Abstract
Polymerization while swelling, a promising and high-efficiency technique based on seeded polymerization, was applied to synthesize monodisperse polystyrene (PS) microspheres with an average diameter of ∼6 μm. The polymerization time was reduced to only 22 hours compared to the traditional way of about 3 days. Taking the synthesized PS particles as templates, conductive shells were prepared by chemically plating nickel on the particle surface. Moreover, an anisotropic conductive film (ACF) was produced using conductive nickel microspheres as electricity transmission media. After removing the PS templates, the obtained magnetic nickel hollow microspheres have a potential application in rapid waste removal and detoxification extraction with a very simple procedure.
Introduction
Due to the large specific surface area and uniform size, monodisperse polymer microspheres have been applied in many areas, such as biomedical engineering,1,2 microelectronics,3 optical devices, separation and purification.4,5 Over the past few decades, researchers have produced monodisperse polymer microspheres using dispersion polymerization,6,7 seeded polymerization,8–11 precipitation polymerization,12,13 and microfluidic methods.14 Among those, seeded polymerization is often adopted to synthesise polymer microspheres with diameters from 1 μm to 40 μm. However, the traditional seed polymerization is time consuming due to the separated steps of swelling and polymerization.Recently, magnetic and conductive microspheres of polymer and metal have attracted great interest.15–19 Many synthetic methods have been introduced to produce the polymer/metal core–shell microspheres.20–25 Their applications in anisotropic conductive film (ACF) have been investigated.26 ACF has been extensively used in flat panel displays and electronic circuits over the past thirty years.27–32 In an ACF material, conductive microspheres disperse in a thin layer of polymeric binder to realize the anisotropic electric conductive functions.33
Another important application of the polymer/metal core–shell microspheres is making metal hollow spheres. For example, Goedel et al. synthesized hollow particles of nickel–cobalt alloys based on poly(methyl methacrylate)/metal alloy core–shell microspheres.34 As special derivates of core–shell materials, hollow spheres have been applied in many fields, such as molecular catalysis, medicine release, photonic crystal, electronic device, separation and purification.35–39
In this paper, monodisperse polystyrene (PS) seed microspheres with an average diameter of 3.0 μm were prepared by dispersion polymerization, and then, larger PS microspheres with an average diameter of ∼6 μm were obtained by a modified seeded polymerization in which swelling and polymerization carried out at the same time to save time and improve efficiency. PS/nickel (Ni) core–shell particles were subsequently prepared by chemical plating method. Finally, Ni hollow microspheres were obtained after removing the PS core by calcination. The application of the obtained PS/Ni core–shell particles and Ni hollow microspheres in ACF and water treatment were studied and discussed, respectively.
Experimental
Chemicals
Styrene (St, 98%) was purchased from Tianjin Wingtai Chemical Company, and distilled under vacuum before use. Ethanol (99.7%), divinylbenzene (DVB, 55%) and benzoyl peroxide (BPO, 95%) were purchased from Tianjin Chemical Company. Polyvinylpyrrolidone (PVP, K-40) was purchased from Fluka. 2,2-Azobisisobutyronitrile (AIBN, 99.9%) and sodium dodecyl sulfonate (SDS, 99.9%) were purchased from Tianjin Fine Chemical Research Institute. Stannous chloride (SnCl2, 98%), nickel sulphate (NiSO4, 98.5%), sodium acetate (99.8%), and potassium dichromate (K2Cr2O7, 99.8%) were purchased from Tianjin Ruijinte Chemical Company. Sodiumhypophosphite (NaH2PO2, 99%) was bought from Tianjin Hongyan Chemical Company. Granular tin (99.9%) was bought from Tianjin Shentai Chemical Company; trisodium citrate dihydrate (99%) was bought from Jiangsu Qiangsheng Chemical Company. Palladium chloride (PdCl2, 59%) was bought from Shanghai Epiphanius Chemical Reagent Company. SU-8 2100 photoresist was bought from Nanjing Baisiyou Chemical Company. Sulphuric acid (H2SO4, 98%) and γ-butyrolactone (99%) were bought from Aladdin Reagent Company. Sodium hydroxide (NaOH, 96%), ammonium hydroxide (NH3·H2O, 25%) and hydrochloric acid (HCl, 36%) were bought from Laiyang Fine Chemical Company. All the chemicals were used as received unless noted elsewhere.Synthesis of PS seed particles by dispersion polymerization
The monodisperse polystyrene (PS) seed particles were synthesized using dispersion polymerization in ethanol with styrene as monomer, PVP as stabilizer and AIBN as initiator.40 The polymerization was carried out at 70 °C in a 250 mL flask under nitrogen atmosphere. 1.35 g of PVP was added into the flask after dissolved in ethanol, and then 15 g of styrene containing 2 wt% AIBN was added under mechanical stirring at 280 rpm. After polymerization for 24 h, PS seed particles with an average diameter of 3.0 μm were obtained by centrifugation and washing with water for three times. The particles were then dried under vacuum at ambient temperature for further use.Synthesis of PS microspheres by a modified seeded polymerization
A modified seeded polymerization method, featured as polymerization while swelling, was applied to the synthesis of PS microspheres with an average diameter of ∼6 μm in a high efficiency manner. 0.1 g of PS seed particles, 0.05 g of PVP, 0.125 g of SDS and 50 g of deionized water were added into a 250 mL flask under nitrogen atmosphere with mechanical stirring at 280 rpm and 70 °C. At the same time, a solution containing 5 g of styrene, 0.1 g of DVB, 0.1 g of BPO, 0.2 g of SDS and 80 g of distilled water was added into the flask by a rate of 50 μL s−1. After reaction for 5 h at 70 °C, 5 g of styrene, 0.1 g of DVB, 0.1 g of BPO, 0.125 g of SDS and 50 g of distilled water were added into the flask by a rate of 50 μL s−1. After reaction for 5 h at 30 °C, 20 g of distilled water and 2 g of PVP were added into the flask, and then the mixture was reacted at 80 °C for 12 h. Finally, the product was centrifuged and washed by deionized water for three times, and dried under vacuum at ambient temperature.Synthesis of PS microspheres by traditional seeded polymerization
PS microspheres with an average diameter of ∼6 μm were synthesized from the 3.0 μm seed particles using a traditional seeded polymerization.41 Firstly, 0.4 g of PS seed particles, 1.4 g of DBP, 0.24 g of BPO, 0.15 g of SDS and 106 g of deionized water were added into a 250 mL flask under nitrogen atmosphere with mechanical stirring at 125 rpm to form dispersion. The swelling step was carried out at room temperature for 24 h. Secondly, St (5 g), DVB (1 g) and an aqueous solution of PVP (1.5 g in 35 mL of water) were added to the dispersion. The swelling step was continued at room temperature for another 24 h with stirring at 125 rpm. Thirdly, the dispersion was polymerized at 70 °C under nitrogen atmosphere for 24 h with stirring at 125 rpm. Finally, the product was centrifuged and washed by deionized water for three times, and dried under vacuum at ambient temperature.Preparation of PS/Ni core–shell microspheres for ACF
The PS/Ni core–shell microspheres were synthesis by chemical plating Ni on the PS microspheres. Firstly, 2 g of PS particles were dispersed into 100 mL of deionized water by vigorous stirring for 15 minutes. Then 3.0 mL of concentrated H2SO4 was slowly added into the suspension solution under stirring. 12 g of K2Cr2O7 was added into the reaction mixture after increasing the temperature to 70 °C. After reaction for 40 min, the suspension was washed with 0.5 mol L−1 of NaOH aqueous solution and deionised water for three times at 25 °C, and then light yellow microspheres were obtained after filtration. Secondly, the treated microspheres were dispersed into 100 mL of deionised water, and then 1 mL of HCl, 0.4 g of tin granule and 4 g of SnCl2 were added into the dispersion under stirring, respectively. After reaction for 20 min at 25 °C, the microspheres was washed and filtered in the same way as the first step. Thirdly, the obtained microspheres were dispersed into 100 mL of deionised water, and then 1 mL of HCl and 0.08 g of PdCl2 were added into the dispersion under stirring. After reaction for 20 min at 25 °C, the microspheres was washed and filtered in the same way as the first step. Finally, the activated microspheres were dispersed into 10 mL of deionised water. 5 mL of plating solution was added at intervals of 5 min into the dispersion under stirring. After chemical plating for 40 minutes in an ice water bath, the core–shell microspheres were separated by centrifugation, washed with deionized water, and dried under vacuum at ambient temperature. The plating solution was prepared as follows: 1.8 g trisodium citrate dihydrate was dissolved in 10 mL of deionized water. 3.0 g NiSO4 was then added into sodium citrate solution under mechanical stirring. After completely dissolved, 2 g of NaH2PO2 and 1.5 g of sodium acetate were added into the mixture under vigorous mechanical stirring, and then the mixture was diluted to 50 mL with an adjusted pH of 6 by adding ammonium hydroxide to form the plating solution.Different amounts of PS/Ni core–shell microspheres as conductive particles were dispersed homogeneously into the SU-8 2100 photoresist which had been diluted four times by γ-butyrolactone. The dispersion was then degassed under vacuum. Afterwards, the dispersion was spin coated onto electrode surface to form a film of 33 μm thickness. After put another electrode on it and photocrosslinked by 365 nm UV radiation at an intensity of 2.32 mW cm−2 for 3 min, the ACF between two electrodes was formed. The conductivity of the films was measured by a multimeter under ambient temperature.
Preparation of Ni hollow spheres for rapid waste removal
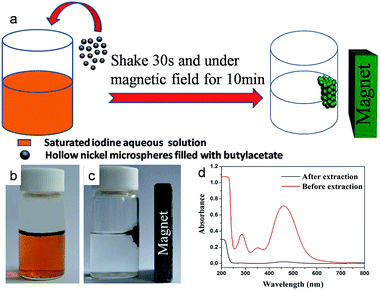
Ni hollow spheres were prepared by immerse the PS/Ni core–shell microspheres followed by a calcination process in air at 400 °C for 5 h to remove the PS/P(S-DVB) templates completely. The obtained Ni hollow microspheres were used for detoxification extraction and rapid waste removal. Iodine was used as a toxic drug analogue to demonstrate the detoxification capability of the core–shell hollow microspheres. 20 mg of the obtained Ni hollow microspheres prefilled with butylacetate (incubated in butylacetate and then filtered) was added into 10 mL of a saturated iodine aqueous solution and shaking intensely for 30 s. The Ni spheres with the extracted wastes were separated in 10 min using a magnet, and the Ni spheres could be recovered after calcination. UV-Vis absorbance of the iodine aqueous solution before and after the detoxification extraction was detected with a Puxi TU-1810 UV-Vis spectrometer.Characterization
The morphology and size of the obtained microspheres were characterized by scanning electron microscopy (SEM, JEOL JSM-6309LV) and transmission electron microscopy (TEM, JEOL JEM-1200). X-ray diffraction (XRD) measurement was performed on a RigakuD/Max2500PC X-ray diffractometer at room temperature. Magnetic measurements of the PS/Ni core–shell nanocomposite hollow microspheres were carried out on an alternate gradient magnetometer (Micro MagTM 2900) at room temperature. The magnetic field was created by a superconducting solenoid in the persistent mode, and the hysteresis loop was recorded in the field up to 4.5 kOe.Results and discussion
Preparation of PS microspheres
A modified seeded polymerization method, featured as polymerization while swelling, is applied to the synthesis of PS microspheres in a high efficiency manner. As shown in Fig. 1, PS microspheres prepared from the traditional and the modified seeded polymerization methods from the same seeds are compared. Fig. 1a shows the PS seeds with an average diameter of 3.0 μm and a polydispersity index (PDI) of 2.86% synthesised by the dispersion polymerization. Fig. 1b shows the PS microspheres with an average diameter of 5.8 μm and a PDI of 3.41% synthesised by the modified seeded polymerization. Fig. 1c shows the PS microspheres with an average diameter of 6.2 μm and a PDI of 3.76% synthesised by traditional seeded polymerization. Compared Fig. 1c with Fig. 1b, we can see PS particles prepared from polymerization while swelling exhibit better monodispersity at the similar final particle size. Additionally, the reaction time for the modified seeded polymerization is only 22 h, while the traditional seeded polymerization takes 72 h. Therefore, the polymerization while swelling technique has a high efficiency in the synthesis of monodisperse PS microspheres with an average diameter of ∼6 μm.As shown in Fig. 2, the size of PS microspheres prepared from the modified seeded polymerization can be varied facilely by changing the mass ratio of seed and monomer. Fig. 2a shows the PS microspheres with an average diameter of 5.4 μm and a PDI of 3.96% synthesised at a seed/monomer ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40. Fig. 2b shows the PS microspheres with an average diameter of 5.6 μm and a PDI of 3.82% synthesised at a seed/monomer ratio of 1
40. Fig. 2b shows the PS microspheres with an average diameter of 5.6 μm and a PDI of 3.82% synthesised at a seed/monomer ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. Fig. 2c shows the PS microspheres with an average diameter of 5.8 μm and a PDI of 3.41% synthesised at a seed/monomer ratio of 1
50. Fig. 2c shows the PS microspheres with an average diameter of 5.8 μm and a PDI of 3.41% synthesised at a seed/monomer ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60. Since the 5.8 μm microspheres have the best monodispersity, they are selected for the preparation of PS/Ni core–shell microspheres.
60. Since the 5.8 μm microspheres have the best monodispersity, they are selected for the preparation of PS/Ni core–shell microspheres.
 | ||
Fig. 2 SEM images of PS microspheres prepared at seed/monomer mass ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (a), 1 40 (a), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (b), and 1 50 (b), and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 (c). Corresponding size distribution were shown in (d), (e) and (f), respectively. 60 (c). Corresponding size distribution were shown in (d), (e) and (f), respectively. | ||
Preparation of PS/Ni core–shell microspheres for ACF
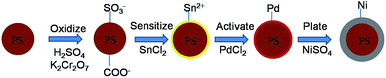
As shown in Fig. 3, the PS/Ni core–shell microspheres are prepared by chemical plating of Ni on the PS microspheres.42 Firstly, the PS microspheres were surface modified by H2SO4 and K2Cr2O7, and then the spheres were sensitized by absorption Sn2+ on the negative charged surfaces. Secondly, the sensitized PS microspheres were activated by PdCl2. In this process, the PdCl2 was reduced to palladium by Sn2+, and an adsorbed layer of Pd was created on the surface of the PS microspheres. During the chemical plating process, Ni2+ was reduced to form a nickel layer on the surface of the PS microspheres under a pH of 6 and a temperature of ∼0 °C using the Pd layer as catalyst.Fig. 4a shows the SEM image of the obtained PS/Ni core–shell microspheres with an average diameter of 6.4 μm. Compared with the bare PS microspheres before plating (Fig. 2c), the diameter increases about 0.6 μm after plating. Thereby, the thickness of the Ni shell is about 0.3 μm. As shown in Fig. 4b, the obtained PS/Ni core–shell microspheres have rough surfaces. Fig. 4c shows the TEM image of the obtained Ni hollow spheres after removing the PS cores by calcination process. The average diameter of the obtained hollow spheres is 6.4 μm, which is the same as that of core–shell microspheres characterized by SEM. The Ni shells are not broken in the removal of PS templates, which indicates the Ni shells are robust and have porous structures. Fig. 4d shows the XRD pattern of the obtained Ni hollow microspheres. The characteristic peaks at 45°, 52° and 76° come from (111), (200) and (220) planes of Ni, respectively. These indicate the nickel shell has very good stability and is not oxidized during the calcination process.
To study the calcination process, TGA was performed on the pure PS microspheres, the PS/Ni core–shell microspheres and the obtained Ni hollow microspheres. The pure PS microspheres were fully decomposed after calcination (Fig. 5a). After being enwrapped and protected by the Ni shells, the decomposition speed of the PS templates slows down, but an earlier weight loss occurs due to the degradation of residual plating reactants in the samples (Fig. 5b). The unchanged weight of the obtained Ni hollow spheres indicates that the PS templates have already been removed completely in the final products (Fig. 5c).
 | ||
| Fig. 5 TGA curves of PS microspheres (a), PS/Ni core–shell microspheres (b), and obtained Ni hollow microspheres (c). | ||
The electric conductivity of the PS/Ni core–shell microspheres is very good with a resistivity of 1.6 × 10−4 Ω m. The magnetic property of the Ni hollow spheres is determined and demonstrated in Fig. 6. The magnetization curves reveal that the Ni hollow spheres have low coercivity and hysteresis in the low field region, indicating that the material exhibits ferromagnetism, because of the magnetic coupling of Ni nanoparticles in the structure. The low coercivity of the Ni hollow spheres originates from the short-range exchange interaction between the adjacent nanoparticles.
As shown in Fig. 7, ACF is an adhesive and curable composite film with monodisperse conductive microspheres dispersed in photoresists. The characteristic of the ACF is that the electrical conduction is allowed in the vertical direction while it is forbidden in the horizontal direction after pressed and cured. Due to the special function, ACF has great potential in device connection of microelectronics and microwave high frequency communications.43
By dispersing the PS/Ni core–shell microspheres in SU-8 photoresist, ACF with an area of 25 cm2 and thickness of 33 μm was prepared and the electric conductivity was characterized (Fig. 8). Increasing amount of conductive microspheres from 5 wt% to 40 wt% can obviously decrease the vertical resistance of ACF from 1213.2 Ω to 12.8 Ω. However, the horizontal resistance of the ACF is always over 200 MΩ at the different microsphere concentrations. Different directions of the film show different conductivities, which demonstrate the excellent anisotropy of ACF made from the PS/Ni core–shell microspheres. The insets of Fig. 8 show the photos of the ACFs containing 5, 10, 20, 30, and 40 wt% of PS/Ni core–shell conductive microspheres, respectively. Because the color of SU-8 photoresist is light brown and the color of the PS/Ni core–shell conductive microspheres is black, we can see that the color of the ACF becomes darker and darker as the content of PS/Ni core–shell conductive microspheres increases.
Detoxification
For having hollow structures with excellent magnetic property and large cubage, the obtained hollow Ni microspheres can be used for detoxification extraction and rapid removal. As shown in Fig. 9, iodine is used as a toxic drug analogue to demonstrate the detoxification capability of the hollow microspheres. 20 mg of the obtained hollow Ni microspheres prefilled with butylacetate was added into 10 mL of a saturated iodine aqueous solution (Fig. 9b). After shaking intensely for 30 s and separating under a magnetic field for 10 min, the iodine aqueous solution becomes colourless (Fig. 9c). Fig. 9d shows that the UV absorption intensity of the iodine aqueous solution decreases by about 97% at 460 nm after adding microspheres for 30 s. The butylacetate in hollow spheres can extract the iodine from the water, while the Ni shells can provide separation ability by magnetic field. High efficiency and ease of separation by external magnet are the superiority of the magnetic hollow microspheres extraction to traditional solid-phase extraction and liquid–liquid extraction techniques.44 Moreover, the hollow Ni spheres can be recovered and reused after calcination at 300–500 °C. Generally, conventional solid-phase extraction has low adsorption capacity and needs several hours to establish the equilibrium state. Whereas, liquid–liquid phase extraction features high efficiency but it is difficult to separate. In our approach, the advantages of the two technologies were combined together. In addition, further applications of the hollow Ni spheres in chromatograph separation and photonic device fabrication are currently under investigation.Conclusion
In this work, a modified seeded polymerization method featured as polymerization while swelling was applied to synthesize monodisperse PS microspheres with an average diameter of ∼6 μm successfully. The polymerization time was reduced to only 22 hours compared to the traditional way of about 3 days. Nickel metal was then coated on the surface of synthesized PS microspheres by chemical plating to produce conductive and magnetic particles. The ACF made from 5 to 40 wt% conductive PS/Ni core–shell microspheres showed anisotropic conductivities. After removing the PS core by calcination, the obtained magnetic hollow Ni microspheres can be used for rapid waste removal and detoxification extraction with a very simple and efficient procedure.Acknowledgements
This work is financially supported by the National Key Basic Research Development Program of China (973 special preliminary study plan, 2012CB722705), the Natural Science Foundation of China (21375069, 21404065, 21574072), the Natural Science Foundation for Distinguished Young Scientists of Shandong Province (JQ201403), the Project of Shandong Province Higher Educational Science and Technology Program (J15LC20), the Graduate Education Innovation Project of Shandong Province (SDYY14028), the Scientific Research Foundation for the Returned Overseas Chinese Scholars of State Education Ministry (20111568), the Science and Technology Program of Qingdao (1314159jch), the China Postdoctoral Science Foundation (2014M561886, 2015T80695) and the Doctoral Scientific Research Foundation of Qingdao.References
- X. J. Ju, L. L. Liu and R. Xie, Polymer, 2009, 50, 922 CrossRef CAS PubMed.
- G. Peng, C. X. Zhao and B. L. Liu, Appl. Surf. Sci., 2012, 258, 5543 CrossRef CAS PubMed.
- M. Okubo, Z. Q. Wang, T. Yamashita, E. Ise and H. Minami, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 3106 CrossRef CAS PubMed.
- J. G. Hu, Y. M. Zhou and X. L. Sheng, RSC Adv., 2015, 5, 17064 RSC.
- E. Unsal, S. T. Camli, A. Tuncel and S. Senel, J. Appl. Polym. Sci., 2004, 92, 607 CrossRef CAS PubMed.
- S. Sugihara, A. Sugihara, A. J. Ryan, A. L. Lewis and S. P. Armel, J. Am. Chem. Soc., 2011, 133, 15707 CrossRef CAS PubMed.
- P. Chambon, A. Blanazs, G. Battaglia and S. P. Armes, Macromolecules, 2012, 45, 5081 CrossRef CAS.
- H. Kloust, E. Poselt, S. Kappen, C. Schmidtke, A. Kornowski, W. Pauer, H. U. Moritz and H. Weller, Langmuir, 2012, 28, 7276 CrossRef CAS PubMed.
- I. H. Kim, J. S. Shin, I. W. Cheong, J. I. Kim and J. H. Kim, Colloids Surf., A, 2002, 207, 169 CrossRef CAS.
- Y. Z. Du, T. Tomohiro and M. Kodaka, Macromolecules, 2004, 37, 803 CrossRef CAS.
- D. Wang, B. Yu and H. L. Cong, Integr. Ferroelectr., 2013, 147, 41 CrossRef CAS PubMed.
- F. Lime and K. Irgum, Macromolecules, 2009, 42, 4436 CrossRef CAS.
- Q. Yan, Y. E. Bai and Z. Meng, J. Phys. Chem. B, 2008, 112, 6914 CrossRef CAS PubMed.
- W. Li, H. H. Pham, Z. H. Nie, B. MacDonald, A. Guenther and E. Kumacheva, J. Am. Chem. Soc., 2008, 130, 9935 CrossRef CAS PubMed.
- G. Wu, K. L. More, C. M. Johnston and P. Zelenay, Science, 2011, 332, 443 CrossRef CAS PubMed.
- Y. Zhang, H. Wang, S. Kräemer, Y. Shi, F. Zhang, M. Snedaker, K. Ding, M. Moskovits, G. J. Snyder and G. D. Stucky, ACS Nano, 2011, 5, 3158 CrossRef CAS PubMed.
- C. Yang, P. Liu and T. Wang, ACS Appl. Mater. Interfaces, 2011, 3, 1109 CAS.
- J. Shen, Y. Zhu and K. Zhou, J. Mater. Chem., 2012, 22, 545 RSC.
- D. E. Park, H. S. Chae and H. J. Choi, J. Mater. Chem. C, 2015, 3, 3150 RSC.
- Y. Hu, T. Zhao and P. Zhu, RSC Adv., 2015, 5, 58 RSC.
- S. R. Hall, S. A. Davis and S. Mann, Langmuir, 2000, 16, 1454 CrossRef CAS.
- J. H. Pan, X. W. Zhang, J. H. Du, D. D. Sun and J. O. Leckie, J. Am. Chem. Soc., 2008, 130, 11256 CrossRef CAS PubMed.
- L. Zhang, J. J. Wu, Y. X. Wang, Y. H. Long, N. Zhao and J. J. Xu, J. Am. Chem. Soc., 2012, 134, 9879 CrossRef CAS PubMed.
- H. Z. Song, Y. X. Li, J. T. Zeng, G. R. Li and Q. R. Yin, J. Magn. Magn. Mater., 2008, 320, 978 CrossRef CAS PubMed.
- W. Li, T. Qiu and L. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 883 CAS.
- J. Ma, H. Gao and X. Chen, Adv. Mater. Processes, 2011, 194, 643 Search PubMed.
- Z. W. Zhong, J. Electron. Packag., 2005, 127, 29 CrossRef CAS.
- I. Watanabe, T. Fujinawa, M. Arifuku, M. Fujii and Y. Gotoh, Proc. - Int. Symp. Adv. Packag. Mater.: Processes, Prop. Interfaces, 9th, 2004, 11, 16 Search PubMed.
- A. J. Sang, K. Kim, J. H. Kim and S. S. Lee, ACS Appl. Mater. Interfaces, 2011, 3, 2904 Search PubMed.
- D. L. Gao and M. S. Zhan, Appl. Surf. Sci., 2009, 255, 4185 CrossRef CAS PubMed.
- Y. C. Chen, R. L. Liu and C. P. Chang, Adv. Mater. Res., 2011, 46, 1163 CrossRef CAS.
- W. Zhao, Q. Zhang and J. Zhang, Polym. Compos., 2009, 30, 1098 CrossRef CAS PubMed.
- J. Liu, J. Eng. Mater. Technol., 1995, 10, 247 Search PubMed.
- P. Tierno and W. A. Goedel, J. Phys. Chem. B, 2006, 110, 3043 CrossRef CAS PubMed.
- H. X. Li, Z. F. Bian, J. Zhu, D. Q. Zhang, G. S. Li, Y. N. Huo, H. Li and Y. F. Lu, J. Am. Chem. Soc., 2010, 129, 8406 CrossRef PubMed.
- H. L. Cong, D. Wang and B. Yu, J. Colloid Interface Sci., 2009, 411, 41 CrossRef PubMed.
- H. L. Cong, Y. Z. Wang, B. Yu, J. L. Wang and M. M. Jiao, New J. Chem., 2013, 10, 1039 Search PubMed.
- D. Nagao, M. Hashimoto, K. Hayasaka and M. Konno, Macromol. Rapid Commun., 2008, 29, 1484 CrossRef CAS PubMed.
- W. F. Shen, J. G. Tang, R. Q. Yang, H. L. Cong, X. C. Bao, Y. Wang, X. Z. Wang, Z. Huang, J. X. Liu, L. J. Huang, J. Q. Jiao, Q. S. Xu, W. C. Chen and L. A. Belfiore, RSC Adv., 2014, 4, 4379 RSC.
- J. S. Song and M. A. Winnik, Macromolecules, 2005, 38, 8300 CrossRef CAS.
- W. Yang, W. Ming and J. Hu, Colloid Polym. Sci., 1998, 276, 655 CAS.
- P. Tierno and W. A. Goedel, J. Phys. Chem. B, 2006, 110, 3043 CrossRef CAS PubMed.
- A. J. Sang, K. Kim, J. H. Kim and S. S. Lee, ACS Appl. Mater. Interfaces, 2011, 3, 2904 Search PubMed.
- B. Yu, H. Yuan, D. Wang, H. L. Cong, X. D. Xu and S. J. Yang, Colloid Polym. Sci., 2014, 292, 2361 CAS.
| This journal is © The Royal Society of Chemistry 2015 |