Molecular hydrogen binding affinities of metal cation decorated substituted benzene systems: insight from computational exploration†
Abstract
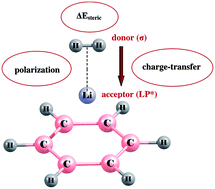
The binding affinity of hydrogen molecules towards Li+ and Mg2+ decorated C6H5X (X = −CH3, −NH2, −CN, −COOH) systems has been investigated theoretically with special emphasis on the nature of the interaction between metal cations and H2 molecules. Our calculations show that binding of H2 over C6H5X−M (where M = Li+, Mg2+) is improved on moving from Li+ to Mg2+. For both C6H5X−M complexes the electron donating substituents weaken the H2 binding energy considerably whereas electron withdrawing substituents slightly strengthen the interaction relative to the C6H6−M complex. The interaction of H2 molecules with the metal centers in Li+ and Mg2+ decorated C6H5X systems has been explored in the light of AIM formalism, NBO analysis and LMOEDA analysis. The polarization and the charge transfer together stabilize the system whereas the pairwise steric exchange interaction renders destabilization of the system. In the case of Mg2+ decorated systems, the amount of charge transfer from the bonding orbital of the hydrogen molecule to the antibonding lone pair orbital of the metal cation and thereby the polarization factor is much higher than that found in corresponding Li+ decorated systems.

 Please wait while we load your content...
Please wait while we load your content...