Expanded graphite/phenolic resin-based carbon composite adsorbents for post-combustion CO2 capture†
Yonggang Jin*a,
Chi P. Huynhb,
Stephen C. Hawkinscd and
Shi Su*a
aCSIRO Energy Flagship, PO Box 883, Kenmore, Qld 4069, Australia. E-mail: yonggang.jin@csiro.au; shi.su@csiro.au; Fax: +61-7-33274455; Tel: +61-7-33274146, +61-7-33274679
bCSIRO Manufacturing Flagship, PMB 10, Clayton, Victoria 3168, Australia
cSchool of Mechanical and Aerospace Engineering, Queen's University Belfast, Belfast, BT9 5AH, UK
dDepartment of Materials Engineering, Monash University, Clayton, Victoria 3800, Australia
First published on 15th July 2015
Abstract
Porous carbon composite adsorbents were prepared from a commercial phenolic resin mixed with a small proportion of thermally expanded graphite (EG) followed by carbonization and physical activation with CO2. The addition of EG dramatically hastens the CO2 activation and results in remarkably enhanced microporosity development in the EG composite compared to the activated phenolic resin alone. The resultant EG composite adsorbents exhibit high CO2 adsorption capacities at 298 K and excellent CO2/N2 adsorption selectivity. In particular, the EG composite shows superior CO2 uptake at low CO2 pressures (47 mg g−1 at 298 K and 0.15 bar), which is more important to actual flue gas applications in post-combustion capture (PCC). Moreover, EG composite adsorbents are especially attractive as the EG component is inexpensive, available in very large amounts and easy to handle and is only required at a low addition level of around 2 wt%. The rapid CO2 activation and the low burn-off for excellent CO2 adsorption performance at low pressures greatly reduce the energy required to produce the adsorbent and the waste generated in activation. This further enhances the cost and environmental advantages of physically activated EG composites over those PCC adsorbents prepared by chemical activation and functionalization of porous carbons. Hence, due to its superior CO2 adsorption properties and favourable fabrication process, the newly developed EG carbon composite adsorbent holds great promise for large-scale deployment and commercial applications to PCC.
1. Introduction
Growing concern with human-induced climate change has attracted widespread efforts to develop cost-effective and robust technologies for post-combustion capture (PCC) of carbon dioxide (CO2) from large point sources such as coal-fired power stations.1 The PCC of CO2 with solid adsorbents is considered a promising alternative to the conventional solvent process which uses chemical absorption with aqueous amine solvents.2,3 Although this is currently the most commercially available PCC technology, the solvent process is energy-intensive and requires strict pre-cleaning treatments to the flue gas. Moreover, it raises environmental concerns due to large volumes of waste water and sludge produced from the solvent process and secondary emissions resulting from the solvent degradation. By contrast, the solid adsorbent process is a low-energy and environmentally-benign technology. It is based on simple physisorption of CO2 with lower heat of CO2 adsorption compared to chemical absorption, which implies potentially lower energy consumption needed for CO2 desorption in adsorbent regeneration. As solid adsorbents are thermally stable and chemically non-reactive with CO2 adsorbates, there are no environmental impacts due to degradation in the adsorbent process. If solid adsorbents are also tolerant of impurities (i.e. SOx and NOx) in the flue gas stream, significant savings in the overall capture cost could be made by removal of flue gas pre-treatments.A large number of solid porous materials have been investigated for CO2 capture including zeolites, porous carbons, functionalized porous silica, metal–organic frameworks and covalent organic frameworks.4–13 Carbonaceous adsorbents have advantages such as low cost and high chemical, thermal and mechanical stability necessary to operate in actual flue gas streams. However, they generally have poor CO2 adsorption capacity, which reduces their cost-effectiveness because more adsorbents are needed to cope with a given flue gas flow and more energy is required to achieve regeneration because more sensible heat is required to heat up a larger amount of adsorbents for thermal regeneration.
Approaches to improving the CO2 adsorption capacity of carbonaceous materials have been mainly focused on (1) development of high-surface-area microporous carbons in a chemical activation method by blending carbon precursors with a very large proportion of harsh chemicals such as KOH followed by pyrolysis;10,14,15 and (2) functionalization of porous carbons with basic groups, such as nitrogen doping and amine loading.16,17 Although the CO2 adsorption capacity is improved, there is a high complexity, cost, waste and inconvenience with chemical activation as large amounts of hazardous chemicals are used and chemical residues have to be thoroughly washed out. The functionalization method also has greater complexity or cost, and some functionalized carbons show difficult and unstable regeneration due to strong interactions with CO2.
The CO2 adsorption capacity of porous carbons can also be improved by physical activation, which entails etching carbons with mild oxidant gases such as CO2 and steam at elevated temperatures, a process that is much more economically and environmentally attractive than chemical activation.18 An important issue with physical activation, particularly for carbon monoliths desirable for practical applications, is mass transport/diffusion of the etchant gases during oxidation.19 Facilitated mass transport of oxidizing molecules within the carbon structure is requisite to boost the reaction of physical activation, thereby enhancing the development of the narrow microporous structure in physically activated carbons for improved CO2 adsorption at ambient conditions.
We recently reported the preparation of carbon composite monoliths with exceptional CO2 adsorption capacity and kinetics, and excellent CO2/N2 selectivity.20 It used a commercial phenolic resin mixed with just 1 wt% of carbon nanotubes (CNTs) and physical activation with CO2. The outstanding performance is attributed to the monolith possessing a hierarchical macroporous–microporous structure with a very high proportion of narrow micropores. The proposed formation mechanism is that the CNTs act as a nanoscale scaffold to establish a macroporous structure in the resin-derived carbon after carbonization, which allows easy access of activating agent CO2 molecules to the monolith's interior. The CNTs also offer a large primary surface area by distributing the resin-derived carbon into micro/nanometre scale domains, thus providing more locations for rapidly creating a large population of narrow micropores in the course of CO2 activation. As a result, the CO2 activation for the CNT-incorporated composite is remarkably quick, exhibiting much greater burn-off than the phenolic resin alone derived carbon at a given period of time and the rapid activation also minimizes pore widening. Therefore, it can be seen that fast mass transport of activating agent molecules is very important to the development of an optimum microporous structure in physically-activated carbons.
In the present study, we incorporated expanded graphite (EG) into phenolic resins to produce carbon composite adsorbents by physical activation with CO2. Compared to CNTs, EG is of lower cost, and easier to make, handle and disperse in the preparation of composites. The large surface area and high aspect ratio (platelet diameter to thickness) of EG is anticipated to function in a somewhat similar manner to CNTs for achieving well-developed narrow microporous structures in the EG/phenolic resin-based carbon composite. The resultant EG monoliths were characterized for surface morphologies, pore structures and gas adsorption properties to understand the effects of EG addition on the development of microporous structures in phenolic resin-derived activated carbons as well as to evaluate CO2 capture performance of EG composites for PCC.
2. Experimental
EG was prepared from commercial expandable graphite flakes (Asbury Carbons, Grade 3772, 80%, >300 μm and carbon content 99%). The expandable graphite flakes were thermally expanded and exfoliated by heating 3 g batches in loosely capped 500 ml single-use tin cans at 1050 °C in a muffle furnace for 3 min. This amount produced approximately 2.5 g of EG which is sufficient to fill the can, which was allowed to cool before opening. The obtained EG powders were well dispersed in an aqueous gel solution containing 5 wt% of methyl cellulose (MC, Aldrich, viscosity (2% in H2O) = 4000 cp).EG carbon composites were prepared by thoroughly mixing 5 g of commercial Novolac phenolic resin (Durez 7716) powder with a given amount of the EG gel paste. The resultant highly viscous mixture was transferred into a cylindrical polypropylene mold (20 mm inner diameter) with five 2 mm diameter channel pins, and then cured at 150 °C for 2 h to solidify the structure. After de-molding, the honeycomb monolith with five channels was carbonized in a tube furnace at 650 °C for 1 h under N2 flow. The carbonized sample was weighted, returned to the tube furnace and heated to 950 °C under N2 flow. At the setpoint temperature, the gas flow was switched to CO2 for a given period to activate the sample with CO2, and then back to N2 as the sample was cooled. The activated sample was weighed to determine the burn-off (defined as (mass of carbonized sample − mass of activated sample)/mass of carbonized sample × 100%) during activation. The activated EG composites were labeled as EG-x-y, where x = the % EG to resin (i.e. mass percentage of EG/phenolic resin) with values of 2, 5 and 10%, and y = the activation time with values of 15 and 30 min. For comparison, the activated sample made of phenolic resin alone without EG was prepared and labeled as Res-60, denoting 60 min activation time.
Sorption measurements for N2 and CO2 were carried out on Micromeritics ASAP 2020 volumetric analyzer at various temperatures after degassing overnight under vacuum at 473 K. The Brunauer–Emmett–Teller (BET) specific surface area (SBET) and the total pore volume (Vt) were obtained from N2 adsorption isotherms at 77 K. SBET calculation was performed below 0.1 relative pressure and Vt was calculated by the N2 amount adsorbed at the relative pressure of about 0.99. Adsorption isotherms for CO2 at 273 K were used to obtain the narrow micropore (<1 nm) size distributions, which were calculated by the density function theory (DFT) method. The surface area (Snm) and the volume (Vnm) of narrow micropores were derived from the above calculations. For comparison, the DFT calculation was also conducted on the N2 adsorption isotherm at 77 K to determine the NMPSD. The heat of CO2 adsorption was calculated using CO2 isotherms at 273, 298 and 323 K based on the Clausius–Clapeyron equation. Morphologies of samples were examined using scanning electron microscopy (SEM, Nava Nano SEM 430) at an operating voltage of 5 kV. The SEM observation was conducted on the sample without coating. Transmission electron microscopy (TEM) was performed on a Tecnai T12 at an operating voltage of 120 kV.
3. Results and discussion
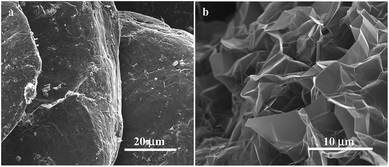
Prior to thermal treatment, the expandable graphite shows a bulky micro-morphology composed of dense graphite flakes (Fig. 1a). Its specified expansion ratio is 270 ml g−1 minimum; however if allowed to expand to this extent, the material is intolerably fluffy and difficult to work with. Thus samples were constrained to a density of 2.5 g to 500 ml or 200 ml g−1. The obtained EG is nevertheless very fluffy and appears in a worm-like particulate shape. As shown in Fig. 1b, EG exhibits an irregular porous network comprising a mass of graphite nanosheets separated by voids and crevices of micron to nanometer. Such morphology is resulted from exfoliation and distortion of graphite nanosheets during rapid thermal expansion and consistent with the observation reported previously.21 The BET surface area (SBET), obtained from N2 adsorption isotherms at 77 K (ESI Fig. S1†), increases from 0.9 m2 g−1 for the expandable graphite to 34.3 m2 g−1 for the EG. In addition, the N2 isotherm of the EG shows a hysteresis loop at relative pressures above 0.5, indicating the presence of mesopores.The surface morphologies of the activated phenolic resin and the EG composite were analyzed by SEM which reveals a dramatic difference after incorporating EG (Fig. 2a–c). The resin alone derived Res-60 exhibits a bulky and dense structure with large vapor holes and little secondary structure such as pores and channels (Fig. 2a). By contrast, the EG composite (EG-5-30) presents a porous microstructure composed of interconnected irregular large particles and shows porosity and channeling at a range of scales from sub-millimeter down (Fig. 2b and c). The original porous network of the EG is covered with resin derived carbons in the EG composite (Fig. 2c), which splits the phenolic resin and subsequently the derived carbon (after carbonization) into small domains that would contribute to the rapid and uniform development of micropores in CO2 activation. The TEM image of the EG composite, as shown in Fig. 2d displays the disordered micropores distributed all over the carbon material, which are responsible for CO2 adsorption under ambient conditions.
 | ||
| Fig. 2 SEM images of (a) the activated phenolic resin Res-60 and (b and c) the EG composite EG-5-30 at different magnifications, and (d) TEM image of EG-5-30. | ||
It can be seen from Table 1 that the composites with EG incorporated are much more reactive with CO2 during activation, exhibiting significant burn-off within much shorter activation duration. Although 7.6 wt% of burn-off was achieved in resin alone derived Res-60 when activated at 950 °C for 60 min, the EG composites took just 30 min to reach the levels of burn-off 2 to 4 times higher. The more EG was incorporated, the higher burn-off was achieved. When the EG composites were activated at 950 °C for 30 min, the burn-off reached 14.3 wt% for EG-2-30, went up to 19.9 wt% for EG-5-30 and doubled to 27.4 wt% for EG-10-30. The dramatic increase of burn-off with the inclusion of EG is likely attributed to the EG's modifications to the microstructure of phenolic resin derived carbons. As discussed above, in contrast to resin alone Res-60, the EG composite has a porous microstructure which would favor mass transport of activating agent CO2 within the monolith, thus leading to rapid burn-off.
| Samples | Burn-off (wt%) | N2 adsorption at 77 K | CO2 adsorption at 273 K | CO2 uptake at 298 K (mg g−1) | |||
|---|---|---|---|---|---|---|---|
| SBET (m2 g−1) | Vt (cm3 g−1) | Snm (m2 g−1) | Vnm (cm3 g−1) | 1 bar, C100 | 0.15 bar, C15 | ||
| Res-60 | 7.6 | 237 | 0.108 | 317 | 0.092 | 82 | 24 |
| EG-2-30 | 14.3 | 723 | 0.290 | 527 | 0.157 | 135 | 45 |
| EG-5-30 | 19.9 | 908 | 0.367 | 477 | 0.143 | 128 | 40 |
| EG-10-30 | 27.4 | 1028 | 0.439 | 413 | 0.125 | 113 | 33 |
| EG-2-15 | 7.6 | 523 | 0.205 | 542 | 0.159 | 127 | 47 |
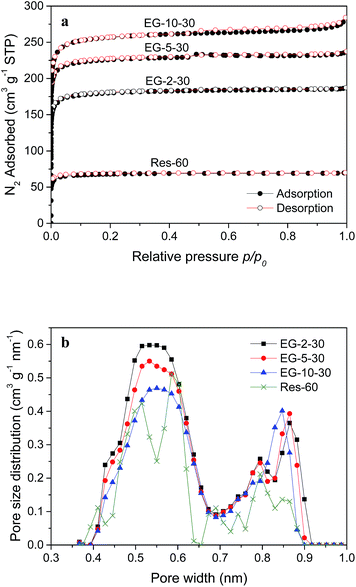
To study the effects of EG addition on the textural properties, N2 and CO2 sorption measurements were carried out at 77 and 273 K, respectively. Fig. 3a shows N2 adsorption–desorption isotherms for the activated phenolic resin (Res-60) and three EG composites activated for 30 min. All the isotherms display significant steep N2 uptake at low relative pressures followed by an adsorption plateau, typical for microporous materials.22 A slight hysteresis for EG-10-30 at relative pressures above 0.5 is similar to the N2 sorption behavior of pure EG (ESI Fig. S1†), suggesting that the EG content in this sample may be excessive and some has failed to disperse effectively. As seen from Table 1, the EG composites exhibit a significantly enhanced porous structure compared to resin alone derived Res-60. The surface area SBET is 237 m2 g−1 for Res-60 and dramatically increased to 723 m2 g−1 for EG-2-30. With more EG incorporated, SBET is increased to 908 m2 g−1 for EG-5-30 and further to 1028 m2 g−1 for EG-10-30. Similarly, pore volumes (Vt) of the composites are remarkably higher than Res-60, and Vt is increased as the addition amount of EG is increased. Therefore, the results clearly indicate that the incorporation of EG greatly enhances the development of microporous structures in the phenolic resin derived carbons.
It is well established that adsorption of CO2 on porous carbons under ambient temperature and pressure conditions is attributed to narrow micropores less than 1 nm.23 Smaller micropore sizes below 0.8 and 0.7 nm have been reported to be responsible for CO2 uptake at atmospheric pressure of 1 bar.22,24,25 The flue gas is typically only 10–15% CO2, i.e. CO2 partial pressure of 0.1–0.15 bar, which suggests that narrow micropores with a even smaller pore size contribute to CO2 adsorption in PCC. Such ultra-narrow micropores are difficult to be detected due to slow diffusion of N2 molecules at 77 K. In this regard, CO2 physisorption at 273 K is more effective to obtain rapid high-resolution characterization of narrow micropores in carbons. For comparison, the narrow micropore (<1 nm) size distribution (NMPSD) of ES-2-30 was obtained from both N2 adsorption isotherm at 77 K (Fig. 3a) and CO2 adsorption isotherm at 273 K (ESI Fig. S2†), as presented in ESI Fig. S3.† Compared to N2 adsorption, features of the NMPSD are captured in much greater detail with the DFT calculation from CO2 adsorption, and pore filling for ultra-small narrow micropores less than 0.5 nm can be observed from CO2 adsorption.
Fig. 3b displays the NMPSDs of the activated phenolic resin and three EG composites obtained from CO2 adsorption isotherms at 273 K (ESI Fig. S2†). The corresponding surface areas (Snm) and pore volumes (Vnm) of narrow micropores are listed in Table 1. It is evident that the EG composites have considerably enhanced development of narrow micropores compared to resin alone derived Res-60, exhibiting higher values of Snm and Vnm. Among the three EG composites activated for 30 min, EG-2-30 contains the least amount of EG but exhibits the highest narrow microporosity in the pore size range below 0.7 nm. It is worth noting that only a small amount of EG addition (2%) to the phenolic resin can dramatically improve the narrow microporosity of the resultant carbon material.
The CO2 equilibrium adsorption capacities at different CO2 partial pressures were obtained from CO2 adsorption isotherms at 298 K (Fig. 4). CO2 molar capacity (mmol g−1) was plotted in Fig. 4 whilst CO2 mass (mg g−1) capacity was listed in Table 1. The values of molar and mass capacity represent mmol and mg of CO2 adsorbed per g of adsorbent, respectively. The CO2 uptake at ambient saturated CO2 pressure of 1 bar was denoted as C100. As noted above, the content of CO2 is low in the flue gas, so in practice CO2 adsorption at a low CO2 partial pressure is a more realistic measure of adsorbent performance for PCC. The CO2 uptake at 0.15 bar corresponding to 15% CO2 partial pressure, denoted as C15, was defined as an indicator of the low-pressure CO2 adsorption capacity. Fig. 4 clearly shows that the EG composite possesses significantly enhanced CO2 adsorption capacities compared to resin alone Res-60 at 298 K and over the measured pressure range up to 760 mmHg. The CO2 uptake of the EG composite declines with an increased incorporation amount of EG. EG-2-30 exhibits the highest CO2 uptake amongst the three EG composites activated for 30 min. The C100 of EG-2-30 (135 mg g−1) is 65% higher than that of resin alone Res-60 (82 mg g−1), while the improvement in the CO2 uptake at the low pressure (C15) is even more striking, increasing by 88% to 45 mg g−1 for EG-2-30 compared to 24 mg g−1 for Res-60 (Table 1). In addition to greatly enhanced CO2 adsorption capacity, sorption of CO2 in the EG composite is reversible and no significant hysteresis in adsorption–desorption curves was observed (Fig. 4). This is desirable for recycle and regeneration of solid adsorbents after CO2 capture.
The above performance of CO2 adsorption is in good agreement with the variation in NMPSDs of the adsorbents as revealed in Fig. 3b. The CO2 uptake in the EG composite is remarkably improved compared to resin alone, which is attributed to a substantial improvement in the narrow microporosity with an incorporation of EG. For a given activation time of 30 min, the narrow microporosity (<0.7 nm) of the EG composite responsible for CO2 uptake at ambient conditions decreases in proportion to the EG content, thereby with EG-2-30 having the largest CO2 uptake among the three EG composites. In addition, there are no correlations between the CO2 uptake of the adsorbents and their textural properties other than characteristics of narrow micropores, such as surface areas SBET and total pore volumes Vt. As listed in Table 1, the more EG the composite contains, the higher values are obtained for both SBET and Vt. Nevertheless, the best CO2 capacity was observed in EG-2-30 containing the least amount of EG.
Based on our previous study,20 the extent of burn-off greatly influences the CO2 capacity of CNT composite adsorbents prepared by CO2 activation. To investigate this effect in the EG composite, we chose the best-performed EG composite containing 2% EG to be activated at 950 °C for 15 min, labeled as EG-2-15. The CO2 adsorption isotherm of EG-2-15 at 298 K is shown in Fig. 5. Compared to EG-2-30, EG-2-15 has much lower burn-off of 7.6 wt%, but improved C15 at 4.7 mg g−1 and reduced C100 at 127 mg g−1 (Table 1). As shown in their NMPSDs (ESI Fig. S4†), EG-2-15 has a larger proportion of micropores below 0.55 nm but less microporosity in the pore size range above 0.55 nm compared to EG-2-30. The variation of the NMPSD well elucidates the increase of C15 and decrease of C100 in EG-20-15. The extended activation widens narrow micropores, which may benefit CO2 uptake at higher CO2 pressures but deteriorate CO2 adsorption at lower pressures. While the narrow micropore size range responsible for CO2 adsorption at 298 K and various pressures cannot yet be defined exactly, it is clear from our results that the C15 value – the specific performance indicator of adsorbents in use for PCC – is most influenced by narrow micropores smaller than about 0.55 nm. This accords with the observation that the CO2 uptake at lower pressures purely depends on the fraction of fine micropores below 0.5 nm for phenolic resin derived activated carbon spheres prepared by KOH chemical activation.22 In addition, with a same level of burn-off as resin alone Res-60, EG-2-15 has dramatically enhanced CO2 uptake, particularly for C15 at 47 mg g−1 almost twice (96% increase) that of Res-60.
Values of up to 135 mg g−1 (3.07 mmol g−1) and 47 mg g−1 (1.06 mmol g−1) were achieved in the EG composites for C100 and C15, respectively. They are lower than those of our previous CNT-incorporated phenolic resin-based composite carbons (maximum C100 at 159 mg g−1 and C15 at 52 mg g−1).20 However, the EG composites outperform other phenolic resin-based activated carbons prepared with organic additives by CO2 activation, in which the C100 up to 108 mg g−1 was obtained.26 Moreover, indicative of CO2 adsorption capacity under the flue gas condition, the C15 exhibited by the EG composite adsorbent is among the highest reported for porous carbons based on recently published comparisons (17–52 mg g−1 at 298 K and 0.15 bar).20,27
As the flue gas is comprised of around 80% nitrogen, a key PCC performance factor is the selectivity of the adsorbent for capturing CO2 over N2. A high CO2/N2 selectivity both increases the overall efficiency by boosting the CO2 uptake, and results in a purer CO2 capture product. CO2 and N2 adsorption capacities of EG-2-15 were compared at 298 K. As shown in Fig. 5, the amount of CO2 adsorbed in EG-2-15 is much higher than that for N2. Its equilibrium CO2/N2 adsorption ratio at 1 bar is 6.0 (2.89 mmol g−1 CO2 vs. 0.48 mmol g−1 N2), close to the ratio 6.3 of our composite with CNTs.20 This value is even superior to those of porous carbons prepared by KOH activation from petroleum pitch (2.8),28 petroleum coke (5.1)29 and sawdust-based chars (5.4).10 In addition, CO2 and N2 adsorption isotherms at 298 K for other three EG composites activated for 30 min were also compared (ESI Fig. S5†). With an increase of EG content, the CO2 uptake is decreased and the amount of N2 adsorbed is also slightly reduced with the N2 uptake at 1 bar being 0.49, 0.47 and 0.41 mmol g−1, respectively for EG-2-30, EG-5-30 and EG-10-30. Similar to that of EG-2-15, the equilibrium CO2/N2 adsorption ratios at 1 bar are around 6.2–6.3 for the above three EG composites.
We also calculated the CO2/N2 adsorption selectivity at 298 K using the ratio of the initial slopes of CO2 and N2 adsorption isotherms at very low pressures (ESI Fig. S6†). EG-2-15 has a high initial selectivity of 18.1. This is comparable to that of the CNT composite (19.8),20 substantially better than reported for pristine microporous carbons (∼7)30,31 and N-doped microporous carbons based on polyimine (12.5)32 and even better than graphene–polyaniline composites (17.9).33 However, the carbon-based adsorbents generally show much lower CO2/N2 adsorption selectivities than recently reported microporous polymer adsorbents such as microporous polyaminals (104)34 and microporous polyimides (56).35 Such remarkable CO2/N2 selectivities are attributed to the abundant CO2-philic functional groups in the microporous polymer in addition to the contribution from the microporous structure.
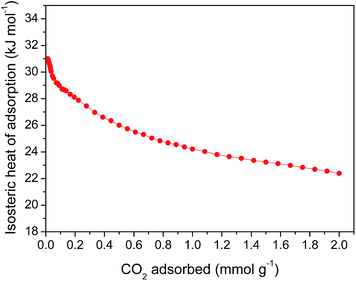
To understand the affinity of the EG composite to CO2 molecules, the isosteric heat of CO2 adsorption for EG-2-15 was calculated from isotherms at 273, 298 and 323 K (ESI Fig. S7†). It is obvious that the CO2 uptake is decreased with an increase of adsorption temperature, which is consistent with the physisorption nature for CO2 adsorption on pristine microporous carbons. At 323 K, the values of C15 and C100 for EG-2-15 are reduced to 32 and 107 mg g−1, respectively. At near zero CO2 loading the isosteric heat is 31.0 kJ mol−1 and declines to 22.4 kJ mol−1 at 2 mmol g−1 of CO2 adsorbed (Fig. 6). The value for EG-2-15 (31.0 kJ mol−1) is slightly lower than that of the CNT composite (32.6 kJ mol−1),20 but higher than for N-free pristine porous carbons (20–30 kJ mol−1).10,36 The heat of adsorption at low surface coverage indicates the interaction between the adsorbent and adsorptive molecules, relying on the pore size distribution in the case of CO2 physisorption on pristine porous carbons by considering the fact that smaller micropores engender stronger interactions between CO2 molecules and porous carbons than larger micropores. Hence, the high heat of CO2 adsorption for EG-2-15 should be attributed to its well-developed narrow microporosity.
4. Conclusions
The addition of a small proportion of EG to the phenolic resin dramatically hastens the CO2 activation and improves the development of microporous structures in resin-derived activated carbons. The resultant EG composite carbons exhibit large pore surface areas and volumes up to more than three times higher than for resin alone. The narrow microporosity responsible for the CO2 uptake at ambient conditions is also remarkably enhanced with the inclusion of EG. The improvement of CO2 uptake in the EG composites is striking with an increase of up to 65% and 96% for C100 and C15, respectively. The EG composites have lower CO2 adsorption capacities than our previous CNT composite carbons but outperform other reported phenolic resin based activated carbons. In particular, they show superior CO2 uptake at low pressures, which is more important to PCC applications. The EG composite adsorbent exhibits excellent CO2/N2 selectivity of 18.1 at 298 K, and a strong affinity to CO2 molecules indicated by its high heat of CO2 adsorption (31.0 kJ mol−1).In addition to their superior CO2 adsorption properties, the physically activated EG composite adsorbents are especially attractive as the EG component is inexpensive, available in very large amounts and easy to handle and is only required at a low addition level of around 2 wt%. The EG component dramatically reduces the activation time and the extent of burn-off necessary to give an excellent PCC adsorbent. This greatly reduces the energy required to produce the adsorbent and the waste generated in activation and further enhances its cost and environmental advantages over chemical activation and functionalization of porous carbons. Therefore, the newly developed EG composite carbon adsorbents are promising for large-scale deployment and commercial applications to PCC.
Acknowledgements
This research was funded by CSIRO. The expandable Graphite (Grade 3772) was donated by Asbury Carbons.References
- D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed.
- Q. Wang, J. Luo, Z. Zhong and A. Borgna, Energy Environ. Sci., 2011, 4, 42–55 CAS.
- A. Samanta, A. Zhao, G. K. H. Shimizu, P. Sarkar and R. Gupta, Ind. Eng. Chem. Res., 2012, 51, 1438–1463 CrossRef CAS.
- S. Choi, J. H. Drese and C. W. Jones, ChemSusChem, 2009, 2, 796–854 CrossRef CAS PubMed.
- Y. F. Zhao, X. Liu and Y. Han, RSC Adv., 2015, 5, 30310–30330 RSC.
- Z. H. Chen, S. B. Deng, H. R. Wei, B. Wang, J. Huang and G. Yu, Front. Environ. Sci. Eng., 2013, 7, 326–340 CrossRef CAS.
- J. Liu, P. K. Thallapally, B. P. McGrail, D. R. Brown and J. Liu, Chem. Soc. Rev., 2012, 41, 2308–2322 RSC.
- F. A. Hasan, P. Xiao, R. K. Singh and P. A. Webley, Chem. Eng. J., 2013, 223, 48–58 CrossRef CAS PubMed.
- X. Y. Ma, Y. Li, M. H. Cao and C. W. Hu, J. Mater. Chem. A, 2014, 2, 4819–4826 CAS.
- M. Sevilla and A. B. Fuertes, Energy Environ. Sci., 2011, 4, 1765–1771 CAS.
- G. P. Hao, W. C. Li and A. H. Lu, J. Mater. Chem., 2011, 21, 6447–6451 RSC.
- Z. J. Zhang, Z. Z. Yao, S. C. Xiang and B. L. Chen, Energy Environ. Sci., 2014, 7, 2868–2899 CAS.
- P. Mohanty, L. D. Kull and K. Landskron, Nat. Commun., 2011, 2, 401 CrossRef PubMed.
- L. K. C. de Souza, N. P. Wickramaratne, A. S. Ello, M. J. F. Costa, C. F. da Costa and M. Jaroniec, Carbon, 2013, 65, 334–340 CrossRef CAS PubMed.
- J. C. Wang, A. Heerwig, M. R. Lohe, M. Oschatz, L. Borchardt and S. Kaskel, J. Mater. Chem., 2012, 22, 13911–13913 RSC.
- G. P. Hao, W. C. Li, D. Qian and A. H. Lu, Adv. Mater., 2010, 22, 853–857 CrossRef CAS PubMed.
- L. Zhao, Z. Bacsik, N. Hedin, W. Wei, Y. H. Sun, M. Antonietti and M. M. Titiric, ChemSusChem, 2010, 840–845 CrossRef PubMed.
- M. Nandi, K. Okada, A. Dutta, A. Bhaumik, J. Maruyama, D. Derks and H. Uyama, Chem. Commun., 2012, 48, 10283–10285 RSC.
- S. R. Tennison, Appl. Catal., A, 1998, 173, 289–311 CrossRef CAS.
- Y. G. Jin, S. C. Hawkins, C. P. Huynh and S. Su, Energy Environ. Sci., 2013, 6, 2591–2596 CAS.
- T. F. Liu, L. Zhao, J. S. Zhu, B. Wang, C. F. Guo and D. L. Wang, J. Mater. Chem. A, 2014, 2, 2822–2829 CAS.
- N. P. Wickramaratne and M. Jaroniec, J. Mater. Chem. A, 2013, 1, 112–116 CAS.
- Z. Zhang, J. Zhou, W. Xing, Q. Xue, Z. Yan, S. Zhuo and S. Z. Qiao, Phys. Chem. Chem. Phys., 2013, 15, 2523–2529 RSC.
- V. Presser, J. McDonough, S. H. Yeon and Y. Gogotsi, Energy Environ. Sci., 2011, 4, 3059–3066 CAS.
- J. P. Marco-Lozar, M. Kunowsky, F. Suárez-García and A. Linares-Solano, Carbon, 2014, 72, 125–134 CrossRef CAS PubMed.
- C. F. Martin, M. G. Plaza, S. Garćia, J. J. Pis, F. Rubiera and C. Pevida, Fuel, 2011, 90, 2064–2072 CrossRef CAS PubMed.
- A. S. González, M. G. Plaza, F. Rubiera and C. Pevida, Chem. Eng. J., 2013, 230, 456–465 CrossRef PubMed.
- A. Wahby, J. M. Ramos-Fernandez, M. Martinez-Escandell, A. Sepulveda-Escribano, J. Silvestre-Albero and F. Rodriguez-Reinoso, ChemSusChem, 2010, 3, 974–981 CrossRef CAS PubMed.
- X. Hu, M. Radosz, K. A. Cychosz and M. Thommes, Environ. Sci. Technol., 2011, 45, 7068–7074 CrossRef CAS PubMed.
- J. D. Carruthers, M. A. Petruska, E. A. Sturm and S. M. Wilson, Microporous Mesoporous Mater., 2012, 154, 62–67 CrossRef PubMed.
- C. Ducrot-Boisgontier, J. Parmentier, A. Faour, J. Patarin and G. D. Pirngruber, Energy Fuels, 2010, 24, 3595–3602 CrossRef CAS.
- J. C. Wang, I. Senkovska, M. Oschatz, M. R. Lohe, L. Borchardt, A. Heerwig, Q. Liu and S. Kaskel, J. Mater. Chem. A, 2013, 1, 10951–10961 CAS.
- K. C. Kemp, V. Chandra, M. Saleh and K. S. Kim, Nanotechnology, 2013, 24, 235703–235710 CrossRef PubMed.
- G. Y. Li, B. Zhang, J. Yuan and Z. G. Wang, Macromolecules, 2014, 47, 6664–6670 CrossRef CAS.
- C. J. Shen and Z. G. Wang, J. Phys. Chem. C, 2014, 118, 17585–17593 CAS.
- S. Himeno, T. Komatsu and S. Fujita, J. Chem. Eng. Data, 2005, 50, 369–376 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09853j |
| This journal is © The Royal Society of Chemistry 2015 |