Luminescence properties and crystal structure of α′-Sr2Si3x/4O2Nx:Eu2+ phosphors with different concentrations of N3− ions
Xiaojun Lia,
Youjie Hua*a,
Hongping Mab,
Degang Denga,
Guohua Jiaa and
Shiqing Xu*a
aCollege of Materials Science and Engineering, China Jiliang University, Hangzhou 310018, China. E-mail: sxucjlu@163.com; Fax: +86-571-28889527; Tel: +86-571-86835781
bSchool of Mechanical & Automotive Engineering, Zhejiang University of Science and Technology, Hangzhou 310012, China
First published on 16th July 2015
Abstract
A series of disordered α′-Sr2Si3x/4O2Nx:Eu2+ (1.333 ≤ x ≤ 2.4) phosphors were synthesized by the conventional solid state reaction method. The disordered α′-Sr2Si3x/4O2Nx:Eu2+ (α′-SSON:Eu2+) phosphors have two distinct activation centers: Eu(I) and Eu(II). With the increase of N concentration, both the luminescence intensity and the dominant peak wavelength (DPWs, which is about 490 nm) of the Eu(I) site were extraordinarily unchanged. In comparison with the yellow emissions (∼580 nm) of the Eu(II) site of the disordered α′-Sr2SiO4:Eu2+, the DPWs of Eu(II) emissions were at red spectral regions (609–618 nm), which depends on the amount of N3−. The PL intensity of the Eu(II) emission band increased first and then decreased, and reached a maximum at x = 2. The disordered α′-SSON is a substitutional solid solution. Compared with the disordered α′-Sr2SiO4, all the lattice constants of disordered α′-SSON became smaller which led to the decrease of the cell volume. The peaks of the Si–N and Sr–N bond could be observed in FT-IR spectra. The Si–(N/O)4 tetrahedrons transformed from Si–O4, Si–NO3, and Si–N2O2 into Si–N3O with the increase of N content. The bond lengths of Si–N and Sr–(N/O) were within the normal ranges compared with other silicon-based oxynitrides. The Si–O bond lengths became shorter due to the extrusion effects of longer Si–N bonds. Both of the average bond lengths of Sr1–(N/O) and Sr2–(N/O) in disordered α′-SSON became longer than that of disordered α′-Sr2SiO4. Due to the red emission and high photoluminescence intensity of the disordered α′-Sr2Si3x/4O2Nx:Eu2+ (1.333 ≤ x ≤ 2.4), we anticipate that these materials can be used as red phosphors in white light emitting diodes.
1 Introduction
The most widely used commercial white LEDs are made of blue GaInN chips and a yellow phosphor (Y1−xGdx)3(Al1−yGay)5O12:Ce3+ (YAG:Ce3+).1 However, they have drawbacks such as low color-rendering index (CRI) and high color temperature because of the deficiency of the green and red compounds. As promising fluorescent materials for white LEDs, rare earth doped nitride and oxynitride phosphors have excellent luminous performances, such as high thermal and chemical stability, a wide effective excitation spectral region, rich luminous color which covers the whole visible region, structural diversity, high quantum efficiency, etc.2–5 A series of these compounds have been synthesized and investigated, such as CaAlSiN3:Eu2+,6–8 M2Si5N8:Eu2+/Ce3+,2,3,9–12 α/β-SiAlONs:Eu2+,13–15 and MSi2O2N2:Eu2+/Ce3+ (M = Ca, Ba, Sr).16–20 The production costs of commercial red phosphors, such as CaAlSiN3:Eu2+ and Sr2Si5N8:Eu2+, are too expensive due to their severe preparation conditions. The preparation of such red phosphors requires not only high temperature and high pressure conditions but also an oxygen-free atmosphere. Therefore, it is urgent to develop a novel high efficient, cost-effective and red emitting phosphor that can be used for white pc-LEDs.As a traditional phosphor, Sr2SiO4:Eu2+ has been extensively studied because of its special structure and tunable light-emitting properties.21–32 Sr2SiO4 has two crystallographic phases: orthorhombic (α′-Sr2SiO4) and monoclinic (β-Sr2SiO4).21–26 Both of them have two luminescence centers: Eu(I) and Eu(II). Eu(I) is ten-coordinated and Eu(II) is nine-coordinated by oxygen atoms within a limited range.27–32 Recently, due to the intense emission in the red spectral range, Sr2SiO4:Eu2+ with N3− substitution has been extensively studied.33–37 Sohn et al. prepared Sr2SiO4−xN2x/3:Eu2+ phosphors through spark plasma sintering (SPS) method and their luminescent properties were investigated.33 Zhao et al. reported the detailed crystal structure of Sr2SiNzO4−1.5z:Eu2+ (0.7 < z < 1.2) by Rietveld refinement and the red emission of this phosphor was attributed to the overlapped two bands due to two distinct Eu(I) and Eu(II) sites.34 (Sr, M)2Si(O1−xNx)4:Eu2+ (M = Ca, Ba, Mg) reported by Kim et al. was considered as a non-stoichiometric solid-solution with the substitution of N3− for O2− and the red emission was assigned to the Eu(II) site.35,36 Kim et al. proposed that the nitridation effect led to a dramatic change in the crystal field surrounding the Eu(II) site but rarely affected the Eu(I) site.36 Ju et al. obtained a strong red-emission in Sr2SiO4:Eu2+ phosphors through the incorporation of a very small amount of nitrogen. They studied the coordination environment of Eu2+ and the interaction mechanism of nitrogen on red-shift emission.37 All the studies focused on the photoluminescence properties and crystal structure. They either simply attributed the red emission to the strong crystal field splitting and the nephelauxetic effect of N3− or used the interaction mechanism of nitrogen and the coordination environment of Eu2+ to explain the red-shift emission.33–37
However, there is no such study that has been done about the influence of N content on the luminescence properties and crystal structure in Sr2SiO4:Eu2+. Unlike previous studies,33–37 in this work, we successfully synthesized the disordered α′-Sr2Si3x/4O2Nx:Eu2+ (1.333 ≤ x ≤ 2.4) phosphors through the conventional solid-state reaction method. We investigated the N3− ions effects on the crystal structural and luminescent properties of α′-SSON:Eu2+ by varying N content, and also interpreted the intensity change and red-shift phenomenon of the disordered α′-SSON:Eu2+.
2 Experimental sections
2.1 Sample preparation
For comparison, the disordered α′-Sr2SiNzO4−1.5z:Eu2+ (0 ≤ z ≤ 1.333) and disordered α′-Sr2Si3x/4O2Nx:Eu2+ (1.333 ≤ x ≤ 2.4) phosphors were synthesized by the conventional solid-state reaction method in a horizontal tube furnace using starting materials of SrCO3 (AR), α-Si3N4 (Alfa 99.9%), and SiO2 (AR). Eu2O3 (99.9%) was added as activator.Raw materials were mixed in an agate mortar and then filled into BN crucibles. The powder mixtures were preheated at 1100 °C for 2 h, and then fired at 1500 °C (with a heating rate of 5 °C min−1) for 6 h, followed by cooling down to 300 °C at a rate of 5 °C min−1 and down to the room temperature in the furnace with the power switched off. In order to prevent samples from being oxidized, all heating and cooling processes were conducted under flowing reduction atmosphere of 95% N2/5% H2.
2.2 Characterization
The phase composition and crystallinity of the synthesized compositions were investigated by the powder X-ray diffraction (Bruker Axs D2 PHASER diffractometer) with Cu Kα radiation (λ = 1.5405 Å) over the angular range of 10° ≤ 2θ ≤ 80°, operating at 30 kV and 10 mA (scanning rate of 1° min−1). The excitation and emission spectra of the phosphors were measured at room temperature on a PL3-211-P spectrometer (HORIBA JOBIN YVON, America) and a 450 W xenon lamp was used as the excitation source. Rietveld refinements on the X-ray diffraction data were performed using the software TOPAS, using the Sr2SiO4 structure as a starting model. The detail atom arrays in the crystal structure of the samples were determined by the software of Diamond basis on the refined data. The nitrogen and oxygen contents (atomic ratios) were measured by energy dispersive spectrometry system (TEAM Apollo XL EDS, EDAX, America). Fourier-transform infrared spectra (FT-IR) were measured on a BRUKER TENSOR 27 spectrophotometer in the range of 400–4000 cm−1 using the KBr pellet (∼2 wt%) method. The morphology of the synthesized phosphors was observed by scanning electron microscopy (FE-SEM, SU8010, HITACHI, Japan). The absorbance spectra of the samples were measured by an ultraviolet-visible-near infrared spectrophotometer (Uv3600) using BaSO4 as a reference in the range of 200–800 nm. All the above measurements were performed at room temperature.3 Results and discussion
3.1 Crystal structure
The XRD patterns of Sr1.98Si3x/4O2Nx:0.02Eu2+ powders prepared with various N content (x = 1.333–2.4) are shown in Fig. 1. When sintered at 1500 °C, it shows that almost all of diffraction peaks matched well with the α′-Sr2SiO4 (JCPDS no. 39-1256) except for a few impurity associated with β-Sr2SiO4 (JCPDS no. 38-0271) phase. In this paper, we abbreviated the α′-Sr1.98Si3x/4O2Nx to α′-SSON. The impurity peak (2θ = 27.85°) of β-Sr2SiO4 phase was marked as β symbol in Fig. 1. With the N content increased from 1.333 to 2.4, the diffraction peak of β phase gradually increased and the degree of crystallinity became worse. These results indicate that a predominant pure α′-SSON phase have been formed accompanied by a small portion of β-SSON phase.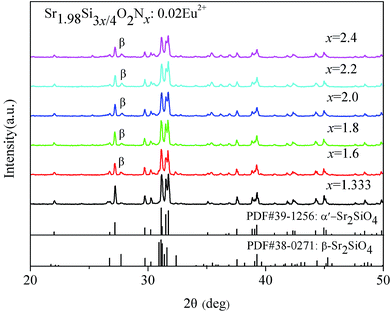 | ||
| Fig. 1 XRD patterns of α′-Sr1.98Si3x/4O2Nx:0.02Eu2+ (1.333 ≤ x ≤ 2.4) powders with different N content. | ||
In order to compare the disordered α′-Sr1.98Si3x/4O2Nx:0.02Eu2+ (1.333 ≤ x ≤ 2.4) phosphors, we also successfully synthesized the disordered α′-Sr1.98SiNzO4−1.5z:0.02Eu2+ (0 ≤ z ≤ 1.333) phosphors through the conventional solid-state reaction method. Fig. 2 shows the XRD patterns of disordered α′-Sr1.98SiNzO4−1.5z:0.02Eu2+ (0 ≤ z ≤ 1.333). These patterns show that almost all of diffraction peaks matched well with the α′-Sr2SiO4 (JCPDS no. 39-1256). These results indicate that a predominant pure α′-Sr1.98SiNzO4−1.5z:0.02Eu2+ (0 ≤ z ≤ 1.333) phase was obtained.
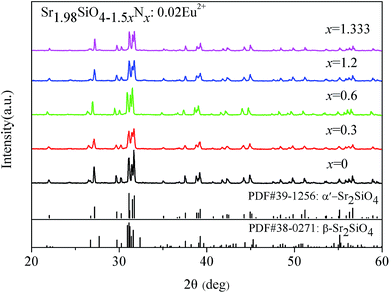 | ||
| Fig. 2 XRD patterns of α′-Sr1.98SiNzO4−1.5z:0.02Eu2+ (0 ≤ z ≤ 1.333) powders with different N content. | ||
Fig. 3 shows the observed (black line), calculated (red line) and difference (green line) XRD profiles for the Rietveld refinement of α′-Sr1.98Si3x/4O2Nx:0.02Eu2+ (x = 2). The peaks (2θ = 27.85°, 32.68°, 36.03°) of β-SSON phase was marked as β symbol in Fig. 2. The actual crystal structure of α′-Sr1.98Si3x/4O2Nx (1.333 ≤ x ≤ 2.4) was analyzed by the software of TOPAS on the basis of the XRD data, using α′-Sr2SiO4 (ICSD: 35666), β-Sr2SiO4 (ICSD: 36041) structure as a starting model. These results indicate that the α′-Sr1.98Si3/2O2N2:0.02Eu2+ host is composed of 92.62% α′-SSON and 7.38% β-SSON phase structures.
 | ||
| Fig. 3 Rietveld refinement XRD patterns of α′-Sr1.98Si3x/4O2Nx:0.02Eu2+ (x = 2) by TOPAS package. (Observed – black line, calculated – red line and difference – green line). | ||
Table 1 only gives the XRD refinement data of the α′-Sr1.98Si3/2O2N2:0.02Eu2+ phase because α′-SSON phase is the main phase of all the samples. These refinement data include cell parameters, wyckoff site, atomic coordinates, occupancy, beq and phase ratios. The α′-Sr2Si3/2O2N2 crystallizes in an orthorhombic unit cell belonging to a space group of Pmnb:ba![[c with combining macron]](https://www.rsc.org/images/entities/i_char_0063_0304.gif) (no. 62). The refinement finally converged to Rexp = 4.64, Rwp = 6.57, Rp = 5.08 and GOF = 1.42, as shown in Table 1. There are obvious differences of the atomic positions and cell volume between the α′-SSO:Eu2+ and α′-SSON:Eu2+ according to ref. 22 and 23.
(no. 62). The refinement finally converged to Rexp = 4.64, Rwp = 6.57, Rp = 5.08 and GOF = 1.42, as shown in Table 1. There are obvious differences of the atomic positions and cell volume between the α′-SSO:Eu2+ and α′-SSON:Eu2+ according to ref. 22 and 23.
Space group: Pmnb:ba![[c with combining macron]](https://www.rsc.org/images/entities/i_char_0063_0304.gif) (orthorhombic) (orthorhombic) |
|
|---|---|
| Cell parameter | Reliability factors |
| a = 5.6663566 Å | Rexp (%) = 4.64 |
| b = 7.0713812 Å | Rwp (%) = 6.57 |
| c = 9.7319768 Å | Rp (%) = 5.08 |
| Cell volume = 389.95026 Å3 | GOF = 1.42 |
| z = 4 | |
| Site | Np | x | y | z | Atom | Occ | Beq |
|---|---|---|---|---|---|---|---|
| Sr1 | 8d | 0.23429 | 0.34045 | 0.57915 | Sr2+ | 0.5 | 0.5578 |
| Sr2 | 8d | 0.26898 | 0.99850 | 0.30157 | Sr2+ | 0.5 | 1.07 |
| Si1 | 4c | 0.25000 | 0.77880 | 0.58320 | Si4+ | 1 | 3.645 |
| O1 | 8d | 0.29751 | 0.98876 | 0.57106 | O2− | 0.5 | 5.02 |
| O2 | 8d | 0.18151 | 0.66957 | 0.42333 | O2− | 0.5 | −3.579 |
| O3 | 8d | 0.50569 | 0.70488 | 0.67266 | O2− | 0.5 | −3.5 |
| O4 | 8d | 0.07687 | 0.73160 | 0.64576 | O2− | 0.5 | 6.309 |
Fig. 4 shows the coordination spheres of the two different Sr2+ sites of the ordered α′-SSO, disordered α′-SSO and disordered α′-SSON (x = 2). Due to the change of O atoms positions and occupation, the α′-SSO phase has two structure model: disordered (isotropic) and ordered (anisotropic) model.23 The O1 and O2 lain on the mirror plane (x = 0.25, occupation = 0.5), but the occupation of O3 and O4 were constrained to be 1 − p and p (p is occupation factor), respectively. In the order α′-SSO model, p = 0, the O1 and O2 lain on the mirror plane (x = 0.25, occupation = 0.5), it has only O3 (occupation = 0.5) but not O4, as shown in Fig. 4(a). In the disordered α′-SSO model, p = 0.5, each atom lying on the mirror plane (x = 0.25) was split into two, in very close positions equivalent by symmetry, and it has O3 (occupation = 0.5) and O4 (occupation = 0.5), as shown in Fig. 4(b). Si had not shifted significantly from the mirror plane, it was constrained at x = 0.25.23
In our experiments, the results of refinement indicate that α′-SSON:Eu2+ presents disordered feature. Fig. 4(c) shows coordination spheres of the two different Sr2+ sites of disordered α′-Sr1.98Si3x/4O2Nx:0.02Eu2+ (x = 2) which is obtained by Rietveld refinement data in Table 1. Zhao et al. had studied detailed crystal structure of Sr2SiNzO4−1.5z:Eu2+ (z ∈ 0.7–1.2) by Rietveld refinement.34 But the distinction of ordered α′-SSON, disordered α′-SSON and β-SSON structures hadn't been effectively distinguished. Owing to the significant difference between the coordination bond length of oxygen and nitrogen, it is feasible to distinguish O and N by XRD refinement data. Fig. 4(b) shows the bond lengths of Sr–O of disordered α′-SSO are evaluated to be in the range of 2.384–3.077 Å. Fig. 4(c) shows the bond lengths of Sr–(N/O) of disordered α′-SSON are evaluated to be in the ranges of 2.357–3.053 Å. The average bond lengths of Sr1–O and Sr2–O in disordered α′-SSO are 2.753 Å and 2.634 Å, respectively. The average bond lengths of Sr1–(N/O) and Sr2–(N/O) in disordered α′-SSON (x = 2) are 2.820 Å and 2.644 Å, respectively.
Apparently, both of the average bond length of Sr1–(N/O) and Sr2–(N/O) in disordered α′-SSON got longer than disordered α′-SSO. The disordered α′-SSON is a substitutional solid solution because the N3− ions substituted for O2− ions. The ionic bond length is the sum of anion and cation radius, so the larger ionic radius will lead to longer ionic bond length. In addition, the ionic radius of N3− (1.32 Å) is larger than O2− (1.24 Å). It can be ascribed to the smaller atomic number, the smaller atomic nuclear mass, and the smaller electronic attraction of N3−. So theoretically, the N3− substitution for O2− could make the average bond length of Sr–O getting longer. Therefore, the results of our experiment are consistent with the theoretical analysis. The Sr–(N/O) bonds in disordered α′-SSON:Eu2+ are between the normal range observed for Sr–O, Sr–N in other oxonitridosilicates or nitrides, such as Sr3Si2O4N2:Sr–O = 2.388–3.157 Å, Sr–N = 2.611–3.096 Å;38 SrSi2O2N2:Sr–O = 1.942–3.029 Å, Sr–N = 2.738–3.064 Å;39 Sr2Si5N8:Sr–N = 2.542–3.231 Å;40 and SrSi7N10:Sr–N = 2.706–3.497 Å.41 However, these results only roughly demonstrate that nitrogen ions have partially been incorporated into the disordered α′-SSO lattice, forming a solid-solution of disordered α′-SSON. In order to further check that the N3− ions have substituted for O2− sites successfully, the formation of disordered α′-SSON will be verified in details by the interpretation of the PL spectra, the analysis of N/O element content, the comparison of FT-IR spectra and the interpretation of Si–(N/O)4 tetrahedrons.
Fig. 5 shows the bond length change of different Sr1/Sr2–O bonds between the disordered α′-Sr2Si1.5O2N2 and disordered α′-Sr2SiO4. As shown in Fig. 5, the bond length of Sr1–O3 and Sr2–O4 of disordered α′-SSON got longer than that of disordered α′-SSO; the bond length of Sr2–O3 of disordered α′-SSON got shorter than that of disordered α′-SSO. Compared with the change of Sr1/Sr2–O1 and Sr1/Sr2–O2, the Sr1/Sr2–O3 and Sr1/Sr2–O4 bond length was changed more obviously due to the substitution of N3−. The bond changes of Sr2–O were more significant than that of Sr1–O. Therefore, we can speculate that N3− was easier to substitute for the site of O3 and O4 atoms. And the Sr2 site was easier to be affected by nitridation, which could be demonstrated in the sections of PL spectra of disordered α′-SSON:0.02Eu2+. But the specific substitution site couldn't be effectively ensured in this article.
 | ||
| Fig. 5 The bond length contrast patterns of Sr1/Sr2–O between the disordered α′-Sr2Si1.5O2N2 and disordered α′-Sr2SiO4. | ||
Table 2 shows the XRD Rietveld refinement of disordered α′-Sr1.98Si3x/4O2Nx:0.02Eu2+ with different N content. Compared with the disordered α′-SSO, all the lattice constants of disordered α′-SSON got smaller which resulted in the decrease of the cell volume. With the increase of N content from 1.333 to 2, the lattice constants and cell volume of disordered α′-SSON decreased first and then increased. It demonstrated that the N3− ions have successfully been introduced into the crystal lattice. The proportion of β-phase is gradually increased from 5.25% to 9.72% with the increase of N content. The specific reasons of the changes of lattice constants and cell volume will be further discussed in the section of Si–(N/O)4 tetrahedrons.
| N(x) | a (Å) | b (Å) | c (Å) | Cell volume (Å3) | Rwp (%) | β-SSO proportion |
|---|---|---|---|---|---|---|
| 1.333 | 5.6722 | 7.0789 | 9.7422 | 391.17 | 6.39 | 5.25% |
| 1.6 | 5.6680 | 7.0738 | 9.7352 | 390.32 | 6.77 | 6.89% |
| 1.8 | 5.6678 | 7.0726 | 9.7340 | 390.20 | 6.49 | 7.27% |
| 2.0 | 5.6664 | 7.0714 | 9.7320 | 389.95 | 6.57 | 7.38% |
| 2.2 | 5.6678 | 7.0740 | 9.7351 | 390.32 | 6.65 | 9.44% |
| 2.4 | 5.6681 | 7.0742 | 9.7357 | 390.38 | 7.71 | 9.72% |
| Disorder α′-SSO | 5.682 | 7.090 | 9.773 | 393.71 |
3.2 Comparison of FT-IR spectra for disordered α′-SSO:Eu2+ and disordered α′-SSON:Eu2+
Fig. 6 presents the FT-IR spectra of disordered α′-SSO:0.02Eu2+, disordered α′-SSON:0.02Eu2+ and Sr2Si5N8:Eu2+ phosphors. Apparently, these FT-IR spectra show remarkable differences between the disordered α′-SSO and disordered α′-SSON. The disordered α′-SSON contained a peak of Sr/Eu–N bond (1363 cm−1),37 while the disordered α′-SSO didn't show such peaks. Besides, the Sr/Eu–N bond (1363 cm−1) in disordered α′-SSON was different from the Sr/Eu–N bond (1209 cm−1) in Sr2Si5N8. With the increase of N content from 1.333 to 2.4, the peak positions of Sr/Eu–N bond (1363 cm−1) had not noticeably altered. While the absorption bands of Si–(N/O)4 tetrahedrons with symmetric and antisymmetric stretching vibration in the range of vibration energy (700 cm−1 to 1100 cm−1) were gradually broadened.42–44 This can be ascribed to the different absorption energy between Si–N and Si–O bond because the longer the bond length, the weaker the bond energy. Then the bond energy of Si–N was less than Si–O due to the longer bond length of Si–N than that of Si–O. With the increase of N content, the quantity of Si–N bond increased simultaneously. Then the left absorption bands of Si–(N/O)4 tetrahedrons were extended toward lower energy. On the other hand, the bond length of Si–O was shortened due to the extrusion effect of the longer Si–N bonds. This led to the results that the right absorption bands of Si–(N/O)4 tetrahedrons were extended toward higher energy position. These results could confirm that nitrogen had entered the crystal lattice and formed chemical bonds with surrounding Sr2+/Eu2+ ions and Si atoms in the disordered α′-SSON:Eu2+ phosphor.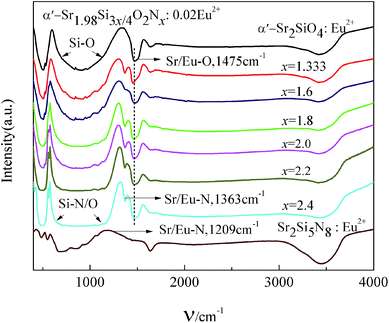 | ||
| Fig. 6 FT-IR spectra of disordered α′-SSO:0.02Eu2+, disordered α′-Sr1.98Si3x/4O2Nx:0.02Eu2+ and Sr2Si5N8:Eu2+ phosphors. | ||
3.3 N/O contents for disordered α′-Sr2Si3x/4O2Nx as a function of x
The N/O contents were measured by energy dispersive spectrometry system (EDS). The measurements and theoretical calculation values of N/O contents are shown in Table 3. With the x values increased from 1.333 to 2.4, the molar ratio of N gradually increased, which is consistent with the theoretical value of the N content. The difference between the experimental and theoretical values is within the limit of error because of the surface oxidation and the measuring error.| Sample | Nitrogen (atomic%) | Oxygen (atomic%) | Theoretical value of the N content (atomic%) |
|---|---|---|---|
| SSON(x = 1.333) | 17.50 | 33.90 | 21.05 |
| SSON(x = 1.6) | 19.93 | 31.46 | 23.53 |
| SSON(x = 1.8) | 21.72 | 27.80 | 25.17 |
| SSON(x = 2.0) | 26.41 | 26.98 | 26.67 |
| SSON(x = 2.2) | 28.57 | 22.26 | 28.02 |
| SSON(x = 2.4) | 30.44 | 20.02 | 29.27 |
3.4 The analysis of SEM images
Fig. 7 shows the SEM images of disordered α′-SSO:Eu2+ and disordered α′-Sr2Si3/2O2N2:Eu2+ phosphors. The powders of disordered α′-SSO and disordered α′-SSON consist of irregular polyhedrons. When sintered at 1500 °C, the particle size of disordered α′-SSO was ∼40 μm due to severe sintering, the particle size of disordered α′-SSON was 15–20 μm. The nitrogen incorporation caused little influence on the morphology of the disordered α′-SSO:Eu2+ phosphors. Obviously, compared with disordered α′-SSO, appropriate particle size of disordered α′-SSON powder could be obtained easily under the high temperature (1500 °C) sintering condition. This is because the optimum synthesis temperature of α′-SSO was ∼1200 °C.26,27 For the disordered α′-SSO, the higher temperature could result in severe sintering. So 1500 °C is too high to get an appropriate particle size in disordered α′-SSO. However, the introduction of N3− would need higher temperature because the break of Si–N bond and the formation of Sr–N bond required higher energy. Therefore, the disordered α′-SSON could get smaller particle size than disordered α′-SSO in the same sintering temperature.3.5 Photoluminescence properties of disordered α′-Sr2−ySi3x/4O2Nx:yEu2+
Fig. 8 shows the PL spectra of disordered α′-SSO:Eu2+ and disordered α′-SSON:Eu2+ (x = 2) phosphors under 300, 360 and 460 nm excitation. The Sr2+ ions in α′-SSO have two sites: Sr1 and Sr2. Sr1 is 10-fold coordiated (CN = 10) whereas Sr2 is 9-fold coordinated (CN = 9) by oxygen atoms within a limited range.23,30–32 Because of the small difference of the ionic radii between Sr2+ (1.31 Å, 9CN; 1.36 Å, 10CN) and Eu2+ (1.30 Å, 9CN; 1.35 Å, 10CN), the Eu2+ will occupy Sr1 and Sr2 sites and form two luminescence center Eu(I) and Eu(II), respectively.45 As shown in Fig. 8, under the 300 nm and 360 nm excitation, the emission peak of Eu(I) position (∼495 nm) had almost no change, whereas the emission peak of Eu(II) position appeared an obvious red-shift which shifted from yellow (∼570 nm) to red spectral ranges (∼612 nm). However, under the 460 nm excitation, the emission peak of Eu(I) site almost disappeared and only Eu(II) site emission could be observed significantly. The emission peak of the Eu(II) position was changed from 578 nm to 616 nm and PL intensity was quite close to the disordered α′-SSO:Eu2+. The specific rules and the reasons for this phenomenon will be discussed in details while studying the effect of N content change in the remainder of this article.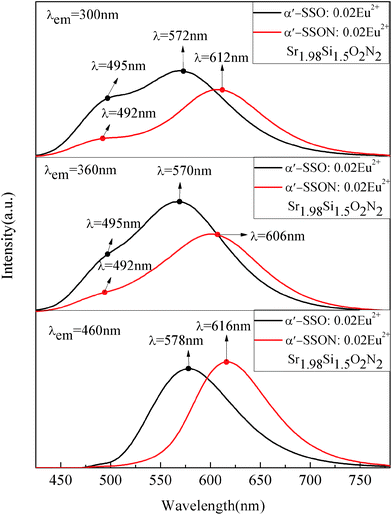 | ||
| Fig. 8 PL spectra comparison of disordered α′-Sr1.98Si2O4:0.02Eu2+ and disordered α′-Sr1.98Si3/2O2N2:0.02Eu2+ under 300, 360 and 460 nm excitation. | ||
According to the emission dominant peak wavelengths (DPWs) of the disordered α′-SSO and α′-SSON, the 490 nm, 580 nm and 616 nm were chosen as the emission wavelengths to test the excitation spectrum of disordered α′-Sr1.98Si3/2O2N2:0.02Eu2+. The corresponding PL excitation (PLE) spectra are shown in Fig. 9. As can be seen from the Fig. 9, compared with blue light excitation (λex = 460 nm), the UV-light excitation (λex = 360 nm) produced more efficient the cyan emission (490 nm) of Eu(I) site.
 | ||
| Fig. 9 Excitation spectra of disordered α′-Sr1.98Si3/2O2N2:0.02Eu2+ corresponded to 490 nm, 580 nm, 616 nm emission, respectively. | ||
The blue light (λem = 460 nm) excitation led to efficient red light emission (615 nm) of Eu(II) site than UV-light excitation. The disordered α′-SSON:0.02Eu2+ (x = 2) could realize an intense red emission at ∼615 nm under 360–500 nm excitation.
Fig. 10 shows the absorption spectra of disordered α′-Sr2Si3x/4O2Nx:0.02Eu2+ (1.333 ≤ x ≤ 2.4) and disordered α′-Sr2SiO4:0.02Eu2+ phosphors. The strong absorption band was located in 250–550 nm in the disordered α′-SSON:Eu2+ and disordered α′-SSO:Eu2+. The disordered α′-SSON:Eu2+ (1.333 ≤ x ≤ 2.4) have stronger absorption than disordered α′-SSO:Eu2+. With the increase of N content, the absorption band was appeared an obvious red-shift, as showed in the shaded area of Fig. 10. This was because the disordered α′-SSON:Eu2+ of longer wavelength emission was easier to be excitated by longer wavelength compared with disordered α′-SSO:Eu2+ of shorter emission, as shown in Fig. 8 and 9.
 | ||
| Fig. 10 Absorption spectra of disordered α′-Sr2Si3x/4O2Nx:0.02Eu2+ (1.333 ≤ x ≤ 2.4), disordered α′-Sr2SiO4:0.02Eu2+ and Sr2Si5N8:Eu2+. | ||
Fig. 11 shows the PL spectra of disordered α′-Sr1.98SiNzO4−1.5z:0.02Eu2+ (0 ≤ z ≤ 1.333). The DPWs of Eu(II) site presented a red-shift and the PL intensity of Eu(II) site of α′-SSON:Eu2+ gradually decreased with the increase of N content from 0 to 1.333.
 | ||
| Fig. 11 PL spectra of disordered α′-Sr1.98SiNzO4−1.5z:0.02Eu2+ (0 ≤ z ≤ 1.333) with varying N content under 460 nm excitation. | ||
Fig. 12 shows the PL spectra of disordered α′-Sr1.98Si3x/4O2Nx:0.02Eu2+ (1.333 ≤ x ≤ 2.4) phosphors. The emission peaks of β phase were not expected to be observed due to its tiny content. The emission peak of Eu(I) site of disordered α′-SSON:Eu2+ was around 490 nm, which was close to the α′-SSO:Eu2+ emission peak of Eu(I) site. It gradually disappeared with the excitation wavelength increased from 300 nm to 460 nm. The emission intensity and position of Eu(I) site was not changed obviously with the increase of N content. However, with the increase of N content from 1.333 to 2.4, the emission peaks corresponding to Eu(II) site in disordered α′-SSON:Eu2+ appeared a significant red-shift. And the emission intensity increased first and then decreased, reaching the maximum at x = 2. These results indicate that Eu(I) positions was not significantly affected by nitridation, whereas Eu(II) positions changed dramatically.
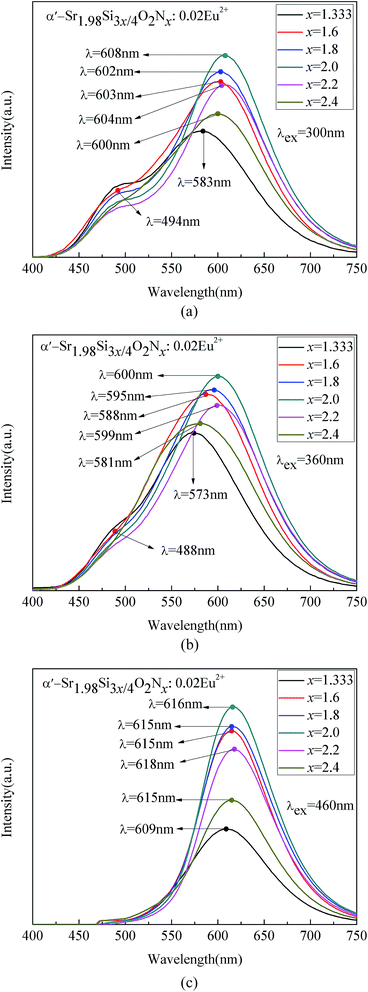 | ||
| Fig. 12 PL spectra of disordered α′-Sr1.98Si3x/4O2Nx:0.02Eu2+ (1.333 ≤ x ≤ 2.4) with varying N content under (a) 300, (b) 360, and (c) 460 nm excitation. | ||
As shown in Fig. 12(c), the emission peak of Eu(I) site almost disappeared whereas Eu(II) site showed significant red light emitting under λex = 460 nm excitation. Compared with the disordered α′-SSO:Eu2+ (λEu(II) = 580 nm), the 580 nm emission bands of disordered α′-SSO:Eu2+ are completely diminished, and a red emission band around 616 nm appeared instead.
Similar to the α′-SSO:Eu2+,46 the PLE spectrum of α′-SSON:Eu2+ consists of two emission bands at ∼490 and ∼616 nm which originate from two luminescence centers: Eu(I) and Eu(II). The ten-coordinated Eu(I) (10-coordiation O atoms, CN = 10) has a loose site and correspond with a higher energy (shorter-wavelength) emission peak at 490 nm. The 9-coordinated Eu(II) (9-coordiation O atoms, CN = 9) has a tight site and correspond with a lower-energy (longer-wavelength) emission peak at 616 nm.29,34,47
The reasons for obvious red shift of Eu(II) site emission can be explained as follows. The partial incorporation of nitrogen ions caused a larger crystal field splitting and gave rise to the nephelauxetic effect due to the covalent bond. It induced a shift of the lowest 5d excitation level toward the ground level of the Eu2+ ions at the Eu(II) site and broadened the PLE spectra, which is a typical 4f7–4f65d1 transition.36,48
The nitridation caused two different spectral variations: the emission peak of Eu(I) site was unchanged while intensity changed slowly and the emission peak of Eu(II) site showed a remarkable red-shift. Kim et al. proposed two possible explanations:35,36 (1) the odds of the N3− ions substitution for O2− ions on Eu(II) position was more than that of Eu(I) position. (2) Two Eu sites have the same N substitution rate. Because of the smaller coordination number (CN) of Eu(II)(CN = 9), the length of Eu(II)–N bond was shorter than Eu(I)–N, which led to the results that Eu(I)(CN = 10) sites of α′-SSON:Eu2+ was not significantly affected by nitridation, whereas the Eu(II) sites was changed dramatically. The dramatic change was because Eu(II) sites was strongly affected by the crystal field that originated from the substituted nitrogen ions.
As shown in Fig. 11, when the substituted nitrogen ions content gradually increased from 0 to 1.333, the luminescence intensity of Eu(II) site gradually reduced, the dominant peak wavelengths (DPWs) emission of Eu(II) showed apparent red-shift because of a stronger crystal field splitting and the nephelauxetic effect. However, as shown in Fig. 12, starting with x = 1.333, when the substituted nitrogen ions content increased from 1.333 to 2.4 continually, the PL intensity of Eu(II) positions raised firstly and then decreased, rather than a continued decrease as it was expected.
3.6 The intensity change of Eu(II) site emission in disordered α′-SSON:Eu2+
Fig. 13 exhibits the transformation of Si-(N/O)4 tetrahedrons in disordered α′-SSON:Eu2+. The bond lengths of Si–(N/O) are in the range of 1.202–1.780 Å. As shown in Fig. 13, during the process of formation of disordered α′-SSON solid-solution, part of the original Si–O4 tetrahedrons gradually transformed into Si–NO3, Si–N2O2 and Si–N3O tetrahedrons with the increase of N-introduction content. | ||
| Fig. 13 Si–(N/O)4 tetrahedrons of the disordered α′-SSON:0.02Eu2+ at (a) disordered α′-SSO (z = 0), (b) x = 1.333, (c) x = 2, (d) x = 2.4. | ||
Table 4 shows the Si–O or Si–N bond lengths with different tetrahedron types in various silicon-based oxynitrides and they were compared with those of disordered α′-SSO and disordered α′-SSON. Obviously, the Si–N bond lengths in disordered α′-SSON are within the normal ranges observed for Si–N bond in other silicon-based oxynitrides.38–41,49,50 However, paradoxically, the Si–O bond lengths in disordered α′-SSON deviated from the normal ranges observed in other silicon-based oxynitrides and got shorter than disordered α′-SSO. Through the introduction of N3− ions, the partial Sr–N and Si–N bonds substituted for Sr–O and Si–O bonds (bond length: Sr–N > Sr–O, Si–N > Si–O). The Si–O bonds were extruded by the longer Si–N bonds and got shorter obviously in the process of forming Si–(N/O)4 tetrahedrons in substitutional solid solution disordered α′-SSON. The Si–O bond lengths of the other silicon-based oxynitrides in Table 4 are within the normal ranges because they are not solid-solutions.
| Silicon-based oxynitrides | Tetrahedron types | Si–O bond lengths (Å) | Si–N bond lengths (Å) |
|---|---|---|---|
| Y4Si2O7N2 (ref. 49) | Si–NO3 | 1.595–1.706 | 1.708, 1.731 |
| Sr3Si2O4N2 (ref. 38) | Si–N2O2 | 1.619–1.657 | 1.660–1.732 |
| SrSi2O2N2 (ref. 39) | Si–N3O | 1.603–1.608 | 1.740–1.746 |
| Si2N2O (ref. 50) | Si–N3O | 1.623 | 1.691–1.750 |
| Disordered α′-SSO | Si–O4 | 1.621–1.655 | |
| Disordered α′-SSON | Si–(N/O)4 | 1.202–1.567 | 1.692–1.780 |
Fig. 14 compares the unit cell volume of disordered α′-SSO:0.02Eu2+ and disordered α′-Sr1.98Si3x/4O2Nx:0.02Eu2+. As shown in Table 2 and Fig. 14, all the lattice constants and cell volume of disordered α′-SSON got smaller than that of disordered α′-SSO, rather than being larger as expected. This is because Si–O bonds were extruded by the longer Si–N bonds and got shorter obviously, as shown in Fig. 13. Due to the mutual extruding effect of the Si–N bonds, with the increase of N content, the Si–O bonds were extruded easily at first and then that would become difficult. Compared with a large amount N3− substituting for O2−, the Si–O bonds was extruded more obviously with a small amount N3− substituting for O2− due to without appearing mutual extruding effect, as show in Fig. 13(a). Therefore, the unit cell volume of disordered α′-SSON:Eu2+ decreased first and then increased with the N-introduction content increased from 1.333 to 2.4.
 | ||
| Fig. 14 Unit cell volume of disordered α′-SSO:0.02Eu2+ and disordered α′-Sr1.98Si3x/4O2Nx:0.02Eu2+ (1.333 ≤ x ≤ 2.4). | ||
As shown in Fig. 13(a) and (b), when the content of nitrogen substitution was less than or equal to 1.333, an asymmetric Si–NO3 tetrahedron was observed in local scale, which undermined the crystal symmetry. At the same time, the degree of crystallinity for disordered α′-SSON:Eu2+ became worse due to the increased proportion of Si–N and (Sr/Eu)–N bonds. Hence, the PL intensity of disordered α′-SSON:Eu2+ gradually declined with the N content increased from 0 to 1.333, as shown in Fig. 11.
As shown in Fig. 13(b) and (c), as the content of nitrogen substitution increased from 1.333 to 2, the Si–(N/O)4 tetrahedrons gradually transformed from asymmetric Si–NO3 tetrahedrons to symmetric Si–N2O2 tetrahedrons and the quantity of (Sr/Eu)–N bonds increased continually. Hence, the proportion of symmetric Si–N2O2 tetrahedrons in the whole crystal increased gradually, and the symmetry degree around Sr2+/Eu2+ sites got better. These results led to an improvement of PL intensity. Furthermore, the optimum crystal symmetry of disordered α′-SSON:Eu2+ was observed when the N/O ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which corresponded to the highest PL intensity of disordered α′-SSON:Eu2+, as shown in Fig. 12. Therefore, the optimal content of N3− ions introduction is x = 2.
1, which corresponded to the highest PL intensity of disordered α′-SSON:Eu2+, as shown in Fig. 12. Therefore, the optimal content of N3− ions introduction is x = 2.
However, Fig. 12 shows that the PL intensity of disordered α′-SSON decrease again when the substituted nitrogen ions content further increased from 2 to 2.4. As shown in Fig. 13(c) and (d), the Si–(N/O)4 tetrahedrons began to transform from symmetric Si–N2O2 tetrahedrons to asymmetric Si–N3O tetrahedrons when the N/O ratio was more than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Meanwhile, the Sr2+/Eu2+ coordinated with more N3− than O2− ions. These facts resulted in a decline of the PL intensity and low crystal symmetry of the entitled phosphors.
1. Meanwhile, the Sr2+/Eu2+ coordinated with more N3− than O2− ions. These facts resulted in a decline of the PL intensity and low crystal symmetry of the entitled phosphors.
3.7 The optimum Eu2+ ions concentration of disordered α′-SSON:yEu2+
Fig. 15 shows the PL spectra of disordered α′-SSON:Eu2+ phosphors with the increase of Eu2+ ions concentration. As can be seen from Fig. 15, with the increase of Eu2+ ions concentration from 0.01 to 0.05, the PL intensity of disordered α′-SSON:yEu2+ raised first and then reduced, reached the maximum at y = 0.02. Therefore, the optimal Eu2+ ions concentration is 2 mol%. | ||
| Fig. 15 PL spectra of disordered α′-Sr2−ySi3/2O2N2:yEu2+ with varying Eu2+ content under 460 nm excitation. | ||
4 Conclusions
We have obtained the disordered α′-SSON:Eu2+ phosphors successfully through the solid state reaction method. Along with the increase of the content of nitrogen introduction from 0 to 2.4 continually, the effects of N3− ions substitution for O2− in α′-SSO:Eu2+ on the structural and luminescent properties was investigated. The disorder of α′-Sr1.98Si3x/4O2Nx:0.02Eu2+ (1.333 ≤ x ≤ 2.4) has been identified to have an orthorhombic structure in the Pmnb:ba![[c with combining macron]](https://www.rsc.org/images/entities/i_char_0063_0304.gif) (no. 62) space group. And it is a substitutional solid solution. Consistent with the theoretical analysis, both of the average bond length of Sr1–(N/O) and Sr2–(N/O) in disordered α′-SSON:Eu2+ became longer than that of disordered α′-SSO:Eu2+. The Si–N and Sr–(N/O) bond lengths in disordered α′-SSON are within the normal ranges observed for Si–N and Sr–(N/O) bond in other silicon-based oxynitrides. The Si–O bond lengths became shorter obviously due to the extrusion effects of longer Si–N bonds. With the increase of N content, the quantity of Sr/Eu–N bonds increased continually and the Si–(N/O)4 tetrahedrons transformed from Si–O4, Si–NO3, and Si–N2O2 into Si–N3O. It led to the result that the PL intensity of Eu(II) sites emission declined in the range of 0–1.333, raised first and then decreased in the range of 1.333–2.4, reaching the maximum at x = 2. The peaks of Si–N and Sr–N bond in disordered α′-SSON:Eu2+ could be observed in FT-IR spectra. The absorption band was appeared an obvious red-shift with the increase of N content. Compared with disordered α′-SSO:Eu2+, with the increase of N content, the Eu(I) site emission peak and intensity of disordered α′-SSON:Eu2+ had hardly change. Whereas the Eu(II) site emission peak of disordered α′-SSON:Eu2+ appeared a obvious red-shift which changed from yellow (∼578 nm) to red (∼618 nm). It can be ascribed to a large crystal field splitting and the increase of nephelauxetic effect. These results indicate that the Eu(I) sites is not significantly affected by nitridation, whereas both of the dominant peak wavelengths (DPWs) and the PL intensity of the Eu(II) emissions changed dramatically due to the effect of N3− introduction. The disordered α′-SSON:Eu2+ can achieve red emission under excitation in the range of 300–500 nm. The optimal PL intensity (x = 2) of the Eu(II) position of disordered α′-SSON:Eu2+ is quite close to disordered α′-SSO:Eu2+. The excellent properties demonstrate that the disordered α′-SSON:Eu2+ phosphors can be applied as red phosphors for white LEDs.
(no. 62) space group. And it is a substitutional solid solution. Consistent with the theoretical analysis, both of the average bond length of Sr1–(N/O) and Sr2–(N/O) in disordered α′-SSON:Eu2+ became longer than that of disordered α′-SSO:Eu2+. The Si–N and Sr–(N/O) bond lengths in disordered α′-SSON are within the normal ranges observed for Si–N and Sr–(N/O) bond in other silicon-based oxynitrides. The Si–O bond lengths became shorter obviously due to the extrusion effects of longer Si–N bonds. With the increase of N content, the quantity of Sr/Eu–N bonds increased continually and the Si–(N/O)4 tetrahedrons transformed from Si–O4, Si–NO3, and Si–N2O2 into Si–N3O. It led to the result that the PL intensity of Eu(II) sites emission declined in the range of 0–1.333, raised first and then decreased in the range of 1.333–2.4, reaching the maximum at x = 2. The peaks of Si–N and Sr–N bond in disordered α′-SSON:Eu2+ could be observed in FT-IR spectra. The absorption band was appeared an obvious red-shift with the increase of N content. Compared with disordered α′-SSO:Eu2+, with the increase of N content, the Eu(I) site emission peak and intensity of disordered α′-SSON:Eu2+ had hardly change. Whereas the Eu(II) site emission peak of disordered α′-SSON:Eu2+ appeared a obvious red-shift which changed from yellow (∼578 nm) to red (∼618 nm). It can be ascribed to a large crystal field splitting and the increase of nephelauxetic effect. These results indicate that the Eu(I) sites is not significantly affected by nitridation, whereas both of the dominant peak wavelengths (DPWs) and the PL intensity of the Eu(II) emissions changed dramatically due to the effect of N3− introduction. The disordered α′-SSON:Eu2+ can achieve red emission under excitation in the range of 300–500 nm. The optimal PL intensity (x = 2) of the Eu(II) position of disordered α′-SSON:Eu2+ is quite close to disordered α′-SSO:Eu2+. The excellent properties demonstrate that the disordered α′-SSON:Eu2+ phosphors can be applied as red phosphors for white LEDs.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant no. 51272243 and 61405185), the Zhejiang Provincial Natural Science Foundation of China (LY14E020008 and LZ14F050001).Notes and references
- S. Nakamura and G. Fasol, The Blue Laser Diode: GaN-Based Light Emitting Diode and Lasers, Springer, Berlin, 1997 Search PubMed.
- X. Piao, T. Horikawa, H. Hanzawa and K. Machidaa, Appl. Phys. Lett., 2006, 88, 1619 CrossRef PubMed.
- Y. Q. Li, J. E. J. van Steen, J. W. H. van Krevel, G. Botty, A. C. A. Delsing, F. J. DiSalvo, G. de With and H. T. Hintzen, J. Alloys Compd., 2006, 417, 273 CrossRef CAS PubMed.
- Y. Kim, J. Kim and S. Kang, J. Mater. Chem. C, 2013, 1, 69 RSC.
- C. Y. Wang, R. J. Xie, F. Li and X. Xu, J. Mater. Chem. C, 2014, 2, 2735 RSC.
- J. W. Li, T. Watanabe, N. Sakamoto, H. S. Wada, T. Setoyama and M. Yoshimura, Chem. Mater., 2008, 20, 2095 CrossRef CAS.
- Y. Q. Li, N. Hirosaki, R. J. Xie, T. Takeda and M. Mitomo, Chem. Mater., 2008, 20, 6704 CrossRef CAS.
- J. Zhu, L. Wang, T. Zhou, Y. Cho, T. Suehiro, T. Takeda, M. Lu, T. Sekiguchi, N. Hirosaki and R. J. Xie, J. Mater. Chem. C, 2015, 3, 3181 RSC.
- X. Piao, T. Horikawa, H. Hanzawa and K. J. Machida, J. Electrochem. Soc., 2006, 153, H232 CrossRef CAS PubMed.
- Y. Q. Li, G. de With and H. T. J. Hintzen, J. Solid State Chem., 2008, 181, 515 CrossRef CAS PubMed.
- S. E. Brinkley, N. Pfaff, K. A. Denault, Z. Zhang, H. T. (Bert) Hintzen, R. Seshadri, S. Nakamura and S. P. DenBaars, Appl. Phys. Lett., 2011, 99, 241106 CrossRef PubMed.
- L. Chen, S. Xue, X. Chen, E. Zhao, J. Deng, X. Deng, S. Chen, Y. Liu, Y. Jiang and H. Li, RSC Adv., 2014, 4, 44317 RSC.
- T. Suehiro, N. Hirosaki, R. J. Xie, K. Sakuma, M. Mitomo, M. Ibukiyama and S. Yamada, Appl. Phys. Lett., 2008, 92, 191904 CrossRef PubMed.
- R. J. Xie, N. Hirosaki, M. Mitomo, T. Suehiro, X. Xu and H. J. Tanaka, J. Am. Ceram. Soc., 2005, 88, 2883 CrossRef CAS PubMed.
- R. J. Xie, N. Hirosaki, K. Sakuma, Y. Yamamoto and M. Mitomo, Appl. Phys. Lett., 2004, 84, 5404 CrossRef CAS PubMed.
- B. Lei, K. Machida, T. Horikawa and H. Hanzawa, Chem. Lett., 2011, 40, 140141 CrossRef.
- Y. Q. Li, G. de With and H. T. J. Hintzen, Chem. Mater., 2005, 15, 4492 RSC.
- J. A. Kechele, O. Oeckler, F. Stadler and W. Schnick, Solid State Sci., 2009, 11, 537 CrossRef CAS PubMed.
- V. Bachmann, C. Ronda, O. Oeckler, W. Schnick and A. Meijerink, Chem. Mater., 2009, 21, 316 CrossRef CAS.
- C. H. Hsu and C. H. Lu, J. Mater. Chem., 2011, 21, 2932 RSC.
- B. G. Hyde, J. R. Sellar and L. Stenberg, Acta Crystallogr., Sect. B: Struct. Sci., 1986, 42, 423 CrossRef.
- M. Catti, G. Gazzoni and G. Ivaldi, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1983, 39, 29 CrossRef.
- M. Catti, G. Gazzoni and G. Ivaldi, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1983, 39, 674 CrossRef.
- M. Catti, G. Gazzoni and G. Ivaldi, Acta Crystallogr., Sect. B: Struct. Commun., 1983, 39, 679 CrossRef.
- L. C. Ju, C. Cai, Q. Q. Zhu, J. Y. Tang, L. Y. Hao and X. Xu, J. Mater. Sci.: Mater. Electron., 2013, 24, 4516 CrossRef CAS.
- A. Nag and T. R. N. Kutty, J. Mater. Chem., 2004, 14, 1598 RSC.
- J. H. Lee and Y. J. Kim, Mater. Sci. Eng., B, 2008, 146, 99 CrossRef CAS PubMed.
- A. Docheon, S. Namsso and D. P. Ki, J. Electrochem. Soc., 2009, 156, 242 Search PubMed.
- Y. S. Won and S. S. Park, J. Phys. Chem. Solids, 2010, 71, 1742 CrossRef CAS PubMed.
- J. K. Han, M. E. Hannah and A. Piquette, J. Lumin., 2012, 132, 106–109 CrossRef CAS PubMed.
- J. S. Kim, P. E. Jeon, J. C. Choi and H. L. Park, Solid State Commun., 2005, 133, 187 CrossRef CAS PubMed.
- H. D. Nguyen, I. H. Yeo and S. Mho, J. Electrochem. Soc., 2010, 28, 167 CAS.
- K. S. Sohn, J. H. Kwak, Y. S. Jung, H. Yan and M. J. Reece, J. Electrochem. Soc., 2008, 155, J58 CrossRef CAS PubMed.
- Z. Zhao, Z. Yang, Y. Shi, C. Wang, B. Liu, G. Zhu and Y. Wang, J. Mater. Chem. C, 2013, 1, 1407 RSC.
- S. J. Lee, S. H. Hong and Y. J. Kim, J. Electrochem. Soc., 2012, 159, J163 CrossRef CAS PubMed.
- J. Park, S. J. Lee and Y. J. Kim, Cryst. Growth Des., 2013, 13, 5204 CAS.
- L. C. Ju, X. Xu, L. Y. Hao, Y. Lin and M. H. Lee, J. Mater. Chem. C, 2015, 3, 1567 RSC.
- X. M. Wang, C. H. Wang, X. J. Kuang, R. Q. Zou, Y. X. Wang and X. P. Jing, Inorg. Chem., 2012, 51, 3540–3547 CrossRef CAS PubMed.
- O. Oeckler, F. Stadler, T. Rosenthal and W. Schnick, Solid State Sci., 2007, 9, 205 CrossRef CAS PubMed.
- T. Schlieper, W. Milius and W. Schnick, Z. Anorg. Allg. Chem., 1995, 621, 1380 CrossRef CAS PubMed.
- G. Pilet, H. A. Höppe, W. Schnick and S. Esmaeilzadeh, Solid State Sci., 2005, 7, 391 CrossRef CAS PubMed.
- R. W. G. Syme, D. J. Lockwood and H. J. J. Kerr, J. Phys. C: Solid State Phys., 1977, 10, 335 CrossRef PubMed.
- J. Etchepare, Spectrochim. Acta, Part A, 1970, 26, 2147 CrossRef CAS.
- P. McMillan, Am. Mineral., 1984, 69, 622 CAS.
- R. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751 CrossRef.
- N. Lakshminarasimhan and U. V. Varadaraju, J. Electrochem. Soc., 2005, 152, H152 CrossRef CAS PubMed.
- K. S. Sohn, B. Lee, R. J. Xie and N. Hirosaki, Opt. Lett., 2009, 34, 3427 CrossRef CAS PubMed.
- P. Dorenbos, J. Lumin., 2000, 91, 155 CrossRef CAS.
- K. J. D. MacKenzie, G. J. Gainsford and M. J. Ryan, J. Eur. Ceram. Soc., 1996, 16, 553 CrossRef CAS.
- C. Brosset and I. Idrestedt, Crystal structure of silicon oxynitride, Si2N2O, Nature Publishing Group, 1964, vol. 4925, p. 1211 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |


