Surface-modified TS-1 with enhanced activity for cyclohexanone ammoximation in a Pickering emulsion and increased stability in hot aqueous ammonia
Lei Xu,
Guojun Lv,
Hang Li,
Yu Shen,
Wangfeng Cai,
Fumin Wang* and
Xubin Zhang*
School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, PR China. E-mail: wangfumin@tju.edu.cn; tjzxb@tju.edu.cn; Fax: +86 22789041; Tel: +86 22789041
First published on 16th July 2015
Abstract
Functional TS-1, partially hydrophobilized with organosilanes, was found to be more active and stable in a Pickering emulsion. In this work, TS-1 behaved simultaneously as a catalyst and solid emulsifier favoring the cyclohexanone ammoximation reaction in organic solvent-free conditions. The composition, structure, formed emulsion morphology, and surface morphology were characterized by Fourier-transform infrared (FT-IR) spectroscopy, conductometer, optical microscope with high speed CCD camera, Brunauer–Emmett–Teller (BET), X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). It indicated that hydrophobilization provided TS-1 a moderate wettability, allowing the formation of a stable Pickering emulsion, thus promoting the performance in catalyzing the cyclohexanone/H2O2/NH3·H2O reaction in the absence of organic solvents. What is more, due to alkyl groups grafted to TS-1, the zeolites were comparatively exclusive to the aqueous phase and they are more capable of enduring severe environment, i.e., 200 °C, under autogenic pressure.
1. Introduction
It is well known that cyclohexanone oxime is an important intermediate for the synthesis of caprolactam, which is crucial to produce nylon-6 fiber and engineering plastic.1–6 With the expansion of the chemical-fiber industry, the demand for cyclohexanone oxime is increasing year by year. A classical route to realize the high yield is using NH3 and H2O2, and ammoximation of cyclohexanone over titanium-containing zeolites carried out in one step, under mild conditions. The liquid phase reaction normally uses tertiarybutanol7 and acetic acid8 as solvents to implement its homogenization so that reaction can be more efficient. But there are still some problems such as solvent recovery and product purification which need to be overcome. As an alternative, emulsification can be an effective option to promote the contact between the reagents. In consideration of the troublesome removal of surfactant, a Pickering emulsion is a good choice.Pickering emulsion, since discovered by Pickering and Ramsden9,10 one century ago, has been widely studied for water/oil and oil/water systems.11 It has a variety use in functional materials, such as capsules,12,13 Janus particles,14,15 composite particles,16,17 and emulsion assemblies.18,19 The solid particles used in Pickering emulsion formation are multitudinous, such as SiO2,20 Fe3O4,21 polyoxometalate nanoparticles,22 and kaolin.23 Things could also happen to catalyst TS-1.
Titanium silicate-1 (TS-1) is the preferred catalyst used in ammoximation of cyclohexanone for its orderly channel and unique shape-selective catalytic function. TS-1, first synthesized by Taramasso et al.24 in 1983, is a typical environmental friendly catalyst. After 1983, TS-1 is synthesize through many ways,25,26 derived from the classic method of Taramosso's. It is an important catalyst for selective oxidation of a large number of organic substrates, for instance, alkanes, alkenes, alcohols, aromatics and phenol.27–33 TS-1 has the potential to act as solid emulsifier, as well, only if it has a moderate wettability.
The stability of zeolite plays an extreme important role in catalyst system, however, according to work of Florian's,34 the framework structure Si–O–Si could been hydrolyzed by hydroxyl ions, especially in harsh environment, e.g. high temperature and pressure. It has been reported that referentially anchoring hydrophobic functionalities on the external surface can effectively prevent direct contact between bulk liquid water and the zeolite, thus preventing the collapse of the framework in hot water.35 Xiangyu Wang36 also a method that after being modified by ethanolamine, TS-1 exhibited excellent catalytic activity and stability in the continuous cyclohexanone ammoximation.
Here, we adjust the wettability of TS-1 by grafting alkyl tails to its surface. Then, the functionalized zeolite acts as both catalyst and solid stabilizer, offering a stable Pickering emulsion environment and catalyzing cyclohexanone ammoximation reaction. The hydrophobilization makes TS-1 partly hydrophobic, resulting in a larger contact area between catalysts and oil phase. Moreover, we investigate the stability of functionalized TS-1 against ammonium hydroxide under high temperature and auto-generated pressure.
2. Experiments
2.1. Materials
TS-1 used in this work was provided by CHINA CATALYST CO., LTD and used as received. The zeolite has a Si/Ti molar ratio of 17, a surface area of 460.7 m2 g−1 and a diameter size of 200 nm. Modification agent used here was octadecyltrichlorosilane (OTS, 95%) provided by J&K, Peking. Solvent here were toluene (BS, Yuanli Chemical Technology Co., China) and acetone (AR, Yuanli Chemical Technology Co., China). Reagents used in reaction were cyclohexanone (AR, Shanghai Civi Chemical Technology Co., China), ammonium hydroxide (25%, AR, Shanghai Civi Chemical Technology Co., China), hydrogen peroxide (20%, AR, Shanghai Civi Chemical Technology Co., China). All reagents were used without further purification.2.2. Functionalization of TS-1
We used a previously described silylation procedure37 for reference to hydrophobilize TS-1. Briefly, in this procedure, 1.5 mL distilled water was dispersed in 50 mL toluene by sonication with a Horn sonicator (AS10200BT), then 1.0 g zeolite was added to the 50 mL toluene, kept stirring for some time at 1000 rpm at 25 °C. A certain amount of octadecyltrichlorosilane in toluene was added to above suspension. The final suspension was stirred for 10 h at 500 rpm at 25 °C. The TS-1 was then collected by centrifugation with a speed of 8000 rpm. After washing several times with acetone, the functionalized zeolite was dried at 100 °C overnight. By changing the amount of OTS, we got a serious of x-OTS-TS-1, which x is indexed as the mass of OTS (mg).2.3. Catalytic activity test
0.80 g TS-1 was dispersed in 5 mL cyclohexanone by sonication with a Horn sonicator (AS10200BT) for 1 min, then 5 mL distilled water was added into the suspension, kept stirring at 700 rpm for 20 min. After that, heated the flake to 70 °C, then NH3·H2O and H2O2 were added drop by drop. The reaction time was 2 h. Reaction result was detected by a gas chromatography.2.4. Stability test
For the stability studies, 1.0 g zeolite was suspended in 30 mL of ammonium hydroxide (12.5%). The mixture was poured into an autoclave with a Teflon liner, and then put at 200 °C under constant agitation. After a specific time, the reaction was quenched by placing the autoclave in air to cool down. The mixture was filtered, and the modified zeolite was washed with distilled water several times. Then the samples were dried in air at 100 °C for 12 h.2.5. Characterization
Infrared radiation (IR) patterns were monitored by a Fourier transform infrared (FTIR) spectroscopy (TENSOR27, Germany Brooke Fourier Infrared Spectrometer Co., Ltd.). Software collected spectra in absorbance mode (wavenumbers from 4000 cm−1 to 400 cm−1) and this spectral range provided obvious changes of hydroxyl groups and alkyl groups. The emulsion phase inversions were observed by a DDS-307 conductometer (Shanghai recision & Scientific Instrument Co., Ltd.) with a Pt platinized electrode at 25 °C. Emulsion morphologies were observed by an optical microscope with high speed CCD camera (AOS X-PRI). For samples prepared, a few of the emulsion was placed on a microscope glass slide as thin as possible and then quickly observed and recorded. Nitrogen adsorption–desorption isotherms were measured at 77 K using a NOVA 2000 series volumetric adsorption system. Powder X-ray diffraction (XRD) patterns were measured on a Bruker D8 X-ray diffractometer with a Cu Kα radiation source (λ = 0.15405 nm) operated at 40 kV and 150 mA. Diffractgrams were obtained with 2θ from 10° to 50° with a scanning rate of 8° min−1. Scanning electron microscopy (SEM) observation was performed on a JEOL S-4800 scanning electron microscope. Transmission electron microscopy (TEM) was performed on a JEOL JEM-2100 transmission electron microscope.3. Results and discussion
3.1. Modification with OTS
IR spectrum is one of the classical methods for structure determination due to its sensitivity to the chemical composition and architecture of molecules. In this work, infrared radiation was conducted to exam whether the functionalization occurred on the external surface of TS-1. The corresponding IR spectra on zeolites before and after functionalization are presented in Fig. 1. It was found there was no adsorption bands appeared at the range of 3000–2750 cm−1 before modification, but after hydrophobilization, new adsorption bands occurred at 2853.15 cm−1 and 2923.11 cm−1, attributing to the symmetrical and asymmetrical stretching vibrations of CH2, respectively. We can also see that with the increase of modifier, the adsorption bands at 2853.15 cm−1 and 2923.11 cm−1 strengthened, indicating the more alkyl tails grafted to the surface of zeolites. | ||
| Fig. 1 IR spectra of different TS-1: (a) TS-1, (b) 12.5-OTS-TS-1, (c) 25.0-OTS-TS-1, (d) 50.0-OTS-TS-1. | ||
3.2. Emulsion properties
The emulsion morphologies of equal volume of cyclohexanone and water were observed and showed in Fig. 3. We can see the morphology of emulsion drops changed with the degree of modification. As the increase of the modification mass of OTS, formed drops became more uniform and regular. The mean drop diameters of each emulsion were calculated and recorded in Fig. 4. The drop diameter of Pickering emulsion built by unfunctionalized zeolite is in average value 180 μm. The drop diameter of Pickering emulsion stabilized by 12.5-OTS-TS-1 is 90 μm, half of the unfunctionalized one. When Pickering emulsion was stabilized by 25.0-OTS-TS-1, the mean drop diameter reduces to 46 μm, 3-folds smaller than the unmodified one. Apparently, alkyl tails anchoring at the surface of zeolites brought a decrease to Pickering emulsion drop size. As a result, a stability enhancement of the formed Pickering emulsion was obtained. It is noteworthy that after the drop diameter reached its minimum, as the hydrophobicity increased, the drop size passed through a sharp maximum and went smaller, due to gravity-induced separation according with the work of Binks's.38
 | ||
| Fig. 3 Morphologies of emulsions stabilized by different TS-1: (a) TS-1, (b) 12.5-OTS-TS-1, (c) 25.0-OTS-TS-1, (d) 37.5-OTS-TS-1, and (e) 50.0-OTS-TS-1. | ||
3.3. Activity in cyclohexanone ammoximation reaction
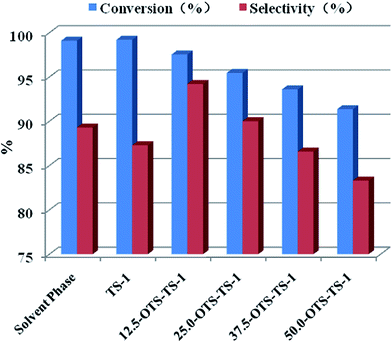
In this part, we compared catalytic activity of unmodified zeolite in both solvent phase (butyl alcohol as organic solvent) and Pickering emulsion, and the results showed that the latter are comparatively acceptable. Although the selectivity of target product cyclohexanone oxime had a tiny decrease, the conversions of cyclohexanone are almost same and there was no organic solvent used. We operated reactions in Pickering emulsion and found that after some degree of hydrophobilized, TS-1 was more active in reaction. The results are shown in Fig. 5. We kept stirring several minutes before adding hydrogen peroxide and ammonia solution, on purpose to form the Pickering emulsion. It turned out that the unmodified TS-1 reached a cyclohexanone conversion of 99% and a selectivity of 87%. Reaction catalyzed by 12.5-OTS-TS-1 got a conversion of 97% and a selectivity of 94% and the one catalyzed by 25.0-OTS-TS-1 got a conversion of 96% and a selectivity of 90%. The reaction catalyzed by catalyzed by 37.5-OTS-TS-1 and 50.0-OTS-TS-1 were even worst. The conversions of them declined to 94% and 91%, simultaneously, the selectivity declined to 87% and 83%. With the increase of modifier, reaction carried out in Pickering emulsion got a decrease of conversion and a maximum of selectivity. There existed a best reaction result catalyzed by 12.5-OTS-TS-1. After that, when the modifier increased, the catalytic activity decreased.Care just taken to the emulsion drop size, the highest catalysis activity should have lied at 25.0-OTS-TS-1 or 50.0-OTS-TS-1, attributing to the small drop size, but it was not the thing. Resulting from the blockage of channel, the chance to get to the active site was reduced. Nitrogen adsorption–desorption isotherms tests show that the unmodified TS-1 has a surface area of 460.7 m2 g−1, while the 12.5-OTS-TS-1 has a surface area of 368.1 m2 g−1, smaller than the former. The surface area of 25.0-OTS-TS-1 is 261.6 m2 g−1 and the surface area of 50.0-OTS-TS-1 is 136.8 m2 g−1. This result gave us the evidence that more modifier, less surface area, and thus lower reaction efficiency.
In the meantime, an interesting phenomenon was observed during the addition of reaction materials. The aqueous contain capacity of formed Pickering emulsion had a negative correlation with modification degree. As far as Pickering emulsion stabilized by 50.0-OTS-TS-1 concerned, water-in-oil Pickering emulsion broke along with the addition of aqueous phase, resulting in the low activity. However, this phenomenon did not occurred when things came to Pickering emulsions stabilized by 12.5-OTS-TS-1 and 25.0-OTS-TS-1. Things above were totally opposite to Binks's work,38 which stated that the ϕw needed for inversion from low conducting water-in-oil emulsion to high oil-in-water emulsion increased with particle hydrophobicity. Possible reason for this was that aqueous phase in our work was consist of ammonium hydroxide and hydrogen peroxide, different from the pure distilled water.
3.4. Stability tests
 | ||
| Fig. 6 XRD patterns of TS-1, Cu2O (upper), and different TS-1 before and after hot ammonia treatment: (a) TS-1, (b) 12.5-OTS-TS-1, (c) 50.0-OTS-TS-1 (lower). | ||
 | ||
| Fig. 7 Relative crystallinity of different TS-1 under different time of hot ammonia hydroxide treatment: (a) TS-1, (b) 12.5-OTS-TS-1, (c) 50.0-OTS-TS-1. | ||
We calculated the relative crystallinity by measuring intensities from samples prepared by mixing the different TS-1 and standard Cu2O together in a known weight ratio39 (the weight of standard added to samples here is constant).
Fig. 6 (lower) shows the X-ray diffraction patterns of TS-1 before and after hot ammonia hydroxide treatment under different degree of modification. We can see that the intensity of unmodified TS-1 changed a lot after treatment, while 12.5-OTS-TS-1 changed less and the 50.0-OTS-TS-1 almost unchanged. It was easy to conclude that the unmodified zeolite endure a loss of crystallinity while the modified ones suffered little.
After treatment with hot ammonia hydroxide, the crystallinity decreased remarkably, indicating that hot ammonia hydroxide damaged the framework of the zeolites. Ascribing to alkyl groups, little damage was noticed. Variation of relative crystallinity of unmodified and hydrophobilized TS-1 at different time were showed in Fig. 7. The intensity ratio of characteristic peaks between MFI (2θ = 23.05°) and Cu2O (2θ = 36.45°) was calculated and the samples without hot aqueous ammonia treatment were assumed to be 100% relative crystalline. After 4 h treatment, the relative crystallinity of zeolite, which was not functionalized, decreased to 0.68. While the 12.5-OTS-TS-1 declined to 0.82, and the 50.0-OTS-TS-1 almost unchanged, it remaining 0.95. And when treating time went up to 8 h and 12 h, relative crystallinity of the unmodified one was down to 0.52 and 0.44, compared to 0.73 and 0.58 of the 12.5-OTS-TS-1, 0.91 and 0.88 of the 50.0-OTS-TS-1. Obviously, as a means to gain better stability in hot aqueous ammonia, functionalizing TS-1 with alkyl moieties was successful.
Table 1 displayed the crystallinity changes of TS-1, which hydrophobilized with a series of modification mass ratio. We calculated the relative crystallinity as point out above and it revealed that the more modifier, the more stability against hot ammonia hydroxide.
| Samples | a | b | c | d | e |
|---|---|---|---|---|---|
| a (a) TS-1, (b) 12.5-OTS-TS-1, (c) 25.0-OTS-TS-1, (d) 37.5-OTS-TS-1, and (e) 50.0-OTS-TS-1. | |||||
| Untreated | 1 | 1 | 1 | 1 | 1 |
| Treated | 0.52 | 0.732 | 0.82 | 0.85 | 0.91 |
| Samples | TS-1 | 25.0-OTS-TS-1 | 50-OTS-TS-1 | |||
|---|---|---|---|---|---|---|
| Treating time (h) | 0 | 8 | 0 | 8 | 0 | 8 |
| Conversion (%) | 99 | 86 | 95 | 92 | 91 | 90 |
| Selectivity (%) | 87 | 54 | 90 | 75 | 83 | 78 |
4. Conclusions
We have modified TS-1 with octadecyltrichlorosilane at facial conditions, and put zeolites hydrophobilized in extreme conditions (200 °C, self-generated pressure) to test its stability, for further regenerability and reusability in cyclohexanone ammoximation catalytic reaction. By hydrophobilizing TS-1 with a certain mass of octadecyltrichlorosilane, their surface wettability changed and thus modified TS-1 can form a stable Pickering emulsion and behave active in cyclohexanone ammoximation even in biphasic emulsion systems and without adding extra organic solvent. What's more, functionalized TS-1 is more immune to hot aqueous ammonia. The grafting alkyl groups on the surface of zeolites help catalyst attaching more with oil phase and hence improve their activity. On the other hand, the hydrophobic groups keep TS-1 distance from aqueous ammonia, as a result, preventing the attack from OH− in aqueous phase. Due to the special structure of MFI topological structure, TS-1 has a strong stability in water, but for hot aqueous ammonia, where exists much more OH−, it does not, especially in hot, high pressure condition. Hydrophobilization can effectively solve this problem. Take the activity and stability both into account, we make a compromise in cyclohexanone ammoximation reaction—moderate stability and activity.Acknowledgements
We gratefully acknowledge the support of the National Basic Research Program of China (973 Program) (Grant no. 2012CB720302) and Program for Changjiang Scholars and Innovative Research Terms in Universities (IRT0936).References
- A. Thangaraj, S. Sivasanker and P. Ratanasamy, J. Catal., 1991, 131, 394–400 CrossRef CAS.
- T. Tatsumi and N. Jappar, J. Catal., 1996, 161, 570–576 CrossRef CAS.
- J. le Bars, J. Dakka and R. A. Sheldon, Appl. Catal., A, 1996, 136, 69–80 CrossRef CAS.
- A. Zecchina, S. Bordiga, C. Lamberti, G. Ricchiardi, C. Lamberti, G. Ricchiardi, D. Scarano, G. Petrini, G. Leofanti and M. Mantegazza, Catal. Today, 1996, 32, 97–106 CrossRef CAS.
- P. Wu, T. Komatsu and T. Yashima, Ammoximation of Ketones over Titanium Mordenite, J. Catal., 1997, 168, 400–411 CrossRef CAS.
- L. Dal Pozzo, G. Fornsari and T. Monti, TS-1, Catal. Commun., 2002, 3, 369–375 CrossRef CAS.
- C. Wu, Y. Wang, Z. Mi, L. Xue, W. Wu, E. Min, S. Han, F. He and S. Fu, Catal. Lett., 2002, 77, 73–81 CAS.
- T. Sooknoi and V. Chitranuwatkul, J. Mol. Catal. A: Chem., 2005, 236, 220–226 CrossRef CAS PubMed.
- S. U. Pickering, J. Chem. Soc., 1907, 91, 2001–2021 RSC.
- W. Ramsden, Proc. R. Soc. London, 1903, 72, 156–164 CrossRef CAS.
- F. Leal-Calderon and V. Schmitt, Curr. Opin. Colloid Interface Sci., 2008, 13, 217–227 CrossRef CAS PubMed.
- A. D. Dinsmore, M. F. Hsu, M. G. Nikolaides, M. Marquez, A. R. Bausch and D. A. Weitz, Science, 2002, 298, 1006–1009 CrossRef CAS PubMed.
- S. Simovic and C. A. Prestidge, Int. J. Pharm., 2006, 324, 92–100 CrossRef PubMed.
- B. Liu, W. Wei, X. Qu and Z. Yang, Angew. Chem., 2008, 120, 4037–4039 CrossRef PubMed.
- D. J. Cole-Hamilton, Science, 2010, 327, 1095–9203 CrossRef PubMed.
- X. Yang, Y. Liang, X. Zhao, Y. Song, L. Hu, X. Wang, Z. Wanga and J. Qiu, RSC Adv., 2014, 4, 31932–31936 RSC.
- H. Gao, Y. Peng, J. Pan, J. Zeng, C. Song, Y. Zhang, Y. Yan and W. Shi, RSC Adv., 2014, 4, 43029–43038 RSC.
- X.-R. Wei, J. Liu, Y. Yang and L. Deng, RSC Adv., 2014, 4, 35744–35749 RSC.
- H. Liu, X. Gu, M. Hu, Y. Hu and C. Wang, RSC Adv., 2014, 4, 16751–16758 RSC.
- B. P. Binks and S. O. Lumsdon, Langmuir, 2000, 16, 2539–2547 CrossRef CAS.
- J. Zhou, X. Qiao, B. P. Binks, K. Sun, M. Bai, Y. Li and Y. Liu, Langmuir, 2011, 27, 3308–3316 CrossRef CAS PubMed.
- L. Leclercq, A. Mouret, A. Proust, V. Schmitt, P. Bauduin, J.-M. Aubry and V. Nardello-Rataj, Chem.–Eur. J., 2012, 18, 14352–14358 CrossRef CAS PubMed.
- L. G. Torres, R. Iturbe, M. J. Snowden, B. Z. Chowdhry and S. A. Leharne, Colloids Surf., A, 2007, 302, 439–448 CrossRef CAS PubMed.
- M. Taramasso, G. Perego and B. Notari, US Pat., 44 10 501, 1983.
- Y. Xue, Y. Wen, H. Wei, M. Liu, X. Huang, X. Ye, X. Wang and B. Li, RSC Adv., 2015, 5, 51563–51569 RSC.
- H. Wei, N. Zhang, T. Zhao, Y. Liu, Y. Wen, X. Wang and B. Li, RSC Adv., 2015, 5, 3642–3647 RSC.
- X. Liu, C. Yang, Y. Wang, Y. Guo, Y. Guo and G. Lu, Chem. Eng. J., 2014, 243, 192–196 CrossRef CAS PubMed.
- H. Li, B. Xu, B. Deng, X. Yan and Y. Zheng, Catal. Commun., 2014, 46, 224–227 CrossRef CAS PubMed.
- Y. Chen, Y. Wu, Y. Zhang, L. Long, L. Tao, M. Yang and N. Tang, J. Mol. Catal. A: Chem., 2012, 352, 102–109 CrossRef CAS PubMed.
- A. Thangaraj, R. Kumar and P. Ratnasamy, J. Catal., 1991, 131, 294–297 CrossRef CAS.
- S. C. Laha and R. Kumar, J. Catal., 2001, 204, 64–70 CrossRef CAS.
- W. Adam, A. Corma, T. I. Reddy and M. Renz, J. Org. Chem., 1997, 62, 3631–3637 CrossRef CAS.
- E. V. Spinace, H. O. Pastore and U. Schuchardt, J. Catal., 1995, 157, 631–635 CrossRef CAS.
- R. M. Ravenelle, S. Florian, A. D'Amico, N. Danilina, J. A. van Bokhoven, J. A. Lercher and C. W. Jones, J. Phys. Chem. C, 2010, 114, 19582–19595 CAS.
- P. A. Zapata, F. Jimmy, M. P. Ruiz, E. J. Rolf and E. R. Daniel, J. Am. Chem. Soc., 2012, 134, 8570–8578 CrossRef CAS PubMed.
- Y. Xue, Y. Xie, H. Wei, Y. Wen, X. Wang and B. Li, New J. Chem., 2014, 38(9), 4229–4234 RSC.
- R. Singh and P. K. Dutta, Microporous Mesoporous Mater., 1999, 32, 29–35 CrossRef CAS.
- B. P. Binks and S. O. Lumsdon, Langmuir, 2000, 16, 8622–8631 CrossRef CAS.
- B. L. Davis, R. Kath and M. Spilde, Powder Diffr., 1990, 5, 76–78 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |