A comprehensive review on the assessment of fuel additive effects on combustion behavior in CI engine fuelled with diesel biodiesel blends
H. K. Imdadul*,
H. H. Masjuki*,
M. A. Kalam*,
N. W. M. Zulkifli,
M. M. Rashed,
H. K. Rashedul,
I. M. Monirul and
M. H. Mosarof
Centre for Energy Sciences, Department of Mechanical Engineering, Faculty of Engineering, University of Malaya, 50603, Kuala Lumpur, Malaysia. E-mail: imdadulduet29@gmail.com; masjuki@um.edu.my; kalam@um.edu.my; Fax: +603 79675317; Tel: +603 79674448
First published on 15th July 2015
Abstract
Development in transport technology is a major issue owing to the increase the number of vehicles, which in turn increases emissions, which result in global warming. The world’s present transportation systems are greatly dependent on petroleum which will deplete rapidly due to limited reserves of fossil fuel. In addition, transportation is responsible for more than 25 percent of the world’s greenhouse gas (GHG) emissions, and this share is rising, which is a threat for future. As an alternative, biodiesel has drawn attention due to its renewability, biodegradability, high conductivity, low sulfur content, flash point, low aromatic content, increased lubricity etc. with less carbon monoxide and carbon dioxide emission. On the other hand, as the viscosity of biodiesel is greater than diesel due to its higher molecular mass and chemical structure, problems such as pumping, combustion, atomization in the injector system, injector deposit, plugging of filters, carbon deposits on piston and head of engine occur. Most previous studies concluded that although particulate emissions from biodiesel fuelled engines are much less than in gasoline, NOx emissions increases significantly. The adjustment of ignition delay in the premixed combustion phase, faster rate of fuel burn, advanced start of combustion, low radiation heat transfer and variable adiabatic flame temperature is mainly responsible for NOx formation and other emissions. Hence fuel additives may play an important role to counteract such problems and achieve various specified standards. Researchers have used many additives to improve the quality of biodiesel such as metal-based additives, oxygenated additives, cetane improvers, ignition promoters, cold-flow improvers, antioxidants and lubricity improvers etc. This literature review characterizes the combustion behavior of diesel engines fuelled by diesel, biodiesel and its blends including additives. It was found that combustion characteristics were improved by introducing additives into diesel and biodiesel blends, while exhaust emissions are also reduced.
1. Introduction
Energy demands during and since the 20th century have drastically increased due to the world’s rapid industrialization, accelerated economic growth, increased human standards of living, modern technology transportation system and power sector1 depending only on limited petroleum or fossil fuel reserves which account for 26–27% total energy consumption and may be replaced completely by biofuels by 2050.2 Oil is the world’s essential wellspring of vitality and chemicals, with a present use of around 12 million tons every day (84 million barrels a day)3 with a projection to 16 million tons every day (116 million barrels a day) by 2030. While 30% of the worldwide oil utilization is for transport, a striking 60% of the rising amount anticipated for 2030 corresponds to transport.4 The combustion of fossil fuels in the transportation sector is the primary source of greenhouse gas and pollutant emissions.5–7 Due to greater fuel economy, higher efficiency, excellent reliability and lower CO2 emissions, diesel engines are widely used. However, burning of such fuel is not environment friendly and produces some other harmful emissions, resulting in global warming, which has led to scientists and researchers to search for renewable alternative fuels for heavy duty diesel engines.8–13 As a renewable fuel biodiesel has been receiving great attention due to its biodegradability, non-toxicity, lower emission, ecofriendly and more reliable behavior, which is produced and formulated from vegetable oil and animal fat.14–16 Also, biodiesel exhibits low aromatic and sulfur contents with high lubrication and octane number.17 However, higher viscosity and large molecular mass of biodiesel leads to lower volatility and poor fuel atomization, injector coking, piston ring sticking, incomplete combustion, problems in cold weather due to adverse impact on cold flow properties1,18,19 and more importantly a significant increase of NOx emission.20,21 Emissions from diesel engines seriously threaten the environment and are considered one of the major sources of air pollution.22 The fourth assessment Report of United Nations Intergovernmental Panel on Climate Change (IPCC) stated that greenhouse gas emissions such as nitrogen oxides (NOx), methane and carbon dioxide (CO2) are the main cause of global warming and an increase in the average global temperature by 2 °C is estimated to result in the deaths of hundreds of millions of people.23 Pollutants also affect ecological systems and creates environmental problems, and produce carcinogenic compounds that lead to significant endangerment of human health. Pollutants from combustion such as oxides of nitrogen (NOx), carbon monoxide (CO), particulate matter (PM), total hydrocarbons (THC), acid rain, and photochemical smog as well as depletion of the ozone layer, has increased concern and driven a number of countries to manage emissions and give directives for implementation and consistency.24Combustion of diesel engine is an important factor which has a great impact on engine performance and emission characteristics. Efficient combustion is desirable, which depends on atomization and evaporation of fuel, blending with surrounding gases, self-ignition, oxidation, turbulence incited by air and fuel stream, the possible interaction of the fuel stream with the cylinder walls, heat exchange between the fuel and the surrounding gases, and between combustion gases and the cylinder walls etc.25 The distinctions in physical and chemical properties of diesel and biodiesel fuels influences the combustion attributes. Due to shorter ignition delay of biodiesel and its blends, the premixed combustion phase occurs earlier compared to neat diesel fuel. Both the premixed combustion phase duration and diffusion combustion phase duration increase with all biodiesel–diesel blends than neat diesel. Higher premixed combustion phase duration of biodiesel–diesel blends is responsible to increase the NOx emission. The maximum rate of pressure rise (ROPRmax) and the maximum heat release rate (HRRmax) of biodiesel are generally lower than that of diesel. Moreover, the brake specific fuel consumption increases somewhat with biodiesel and its blended fuels due to variation in physical properties, combustion and heat release characteristics of biodiesel compared to diesel fuel.26,27
Several experimental studies has been performed to explore the combustion behavior of diesel engine fuelled by biodiesel by altering parameters such as, injection timing, injection pressure, engine load, engine speed, compression ratio, fuel blends etc. Most of these studies concluded that biodiesel fuelled engine showed lower ignition delay and HRR with early start of combustion, increased PM and NOx emission and decreased power loss.28 The combustion phasing29 combustion temperatures,21,30 presence of oxygen content31 and distinctive chemical composition32 of diesel and biodiesel is responsible for this. High viscosity, density and low volatility characteristics of biodiesel resulted in problems in long-term engine performance tests. While combustion quality is influenced by size of fuel molecules, inadequate atomization performance and blocking of fuel entrances in the cylinder are affected by higher viscosity of biodiesel.33,34 Larger chemical structures of biodiesel fuel results in higher viscosity that create problems such as injector coking, ring sticking and gumming in diesel engines.1 Biodiesel also showed increase in PM and NOx emissions. To improve such properties and solve the problems, fuel additives are considered as an alternative and most attractive solution.24 Many studies has been conducted using additives along with diesel, biodiesel and their blends to characterize combustion behavior.35–37 Kinoshita et al.35 reported that the ignition delay of biodiesel with crude glycerine increased the ignition delay (ID). Iranmanesh et al.36 investigated the fuel properties and combustion characteristics of karanja biodiesel in diesel engine with 5%, 10%, 15% and 20% by volume of diethyl ether (DEE), and showed that physicochemical properties such as calorific value, viscosity, specific gravity and liquidity profile were found according to the ASTM standards with improved combustion and cold starting problems. Li et al.37 studied the influences of multifunctional diesel fuel additive with rapeseed oil to aim at improving the combustion performance and concluded that additives diminished the ignition delay (ID), enhanced the premixed combustion and gave better combustion efficiency.
Many investigations have been performed using additives to determine the combustion characteristics and their impact on engine performance and emission with varying load, speed, injection timing and injection pressure. They have also drawn conclusion regarding the effects. The present study emphasized to compare the combustion behavior of diesel engines fuelled by diesel, biodiesel and their blends including additives by reviewing a substantial number of papers and accumulating information from them. It is hoped that an efficient volume of additives with diesel and biodiesel can be formulated and implemented to optimize the combustion with lowest emission and highest performances. This review will also help to further research additives for future investigation.
2. Combustion behaviour in diesel engine
Diesel engines work on the principle of compression ignition. Combustion in a compression ignition (CI) engine is an unsteady process happening at the same time at numerous spots in a non-homogeneous mixture at a rate controlled by fuel injection. The combustion in the compression ignition engine depends on the compression process into the cylinder to increase the temperature and pressure of air so that during fuel injection the mixture of air–fuel auto-ignites. It is necessary to inject the fuel in a fine spray, so that it atomizes and evaporates quickly to mix rapidly with swirling hot air in the combustion chamber. To ensure desired high temperature and pressure for auto-ignition of the mixture to occur into the cylinder, compression ratios of modern CI engines range from 12 to 24.25,38,39The combustion procedure in CI engines can be categorized into three major sections, as shown in Fig. 1.
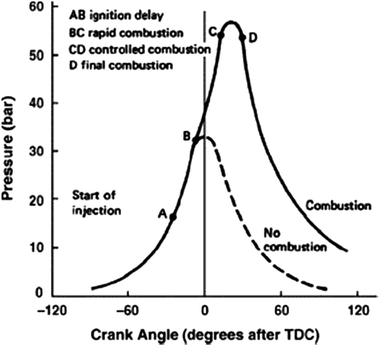 | ||
| Fig. 1 Combustion matters in CI engine.25,39 | ||
2.1. Ignition delay
Among the major combustion parameters, ignition delay is one of the most important combustion properties, which is defined as the time period between the start of injection (SOI) and the start of combustion (SOC) during which each fuel droplet is atomized, vaporized, mixed with air, ignited through auto-ignition, and burned.9,11 The ignition delay period in diesel engine exhibits huge impact on engine design, performance and emission and variation depends on either diesel, biodiesel or their blends in the fuel used. Functionally, the ignition delay (ID) can be divided into two parts, physical delay and chemical delay as shown in Fig. 2.40 | ||
| Fig. 2 Ignition delay mechanism.40 | ||
Physical delay is the period between start of injection and the arrival of chemical reaction conditions. During this time, the fuel is breaking up, vaporized, blended with air and heated to self-ignition temperature. Viscosity represents a significant part of the physical delay of the fuel ignition process and increases as viscosity is increased. During chemical delay time, reactions begin gradually and afterward quicken until combustion occurs. Generally, chemical delay is found to be greater compared to physical delay depending on the temperature of the circumferences. When chemical reactions are quicker at higher temperatures then physical delay becomes greater than the chemical delay.
2.2. Rapid or uncontrolled combustion
During the stages of combustion auto-ignition occurs as some of the mixture has been injected into the cylinder in the ID stage and begins to burn as a premixed charge. As a result a rapid pressure rise is observed depending on the length of ID and mass of fuel present in the combustion chamber.39,412.3. Controlled combustion
By controlling the rate of fuel injected into the cylinder that mixes with compressed hot air, one can control combustion efficiently. As the piston returns away, the in-cylinder mixture cools rapidly due to expansion, resulting in a great decrease in the rates of chemical reaction (often termed as frozen). At such conditions the reaction rates are insignificant, leaving the system that may be far from chemical equilibrium. High levels of NOx and PM are examples of chemical products that are “frozen” well above their equilibrium levels.373. Influence of combustion on performance and emission
Diesel engine combustion is an important but complex phenomenon. The performance and emission of diesel engine depends on combustion efficiency. Many researchers have studied the engine performance, emission and combustion of diesel engine fuelled with biodiesel. Most studies reported that the major factors of combustion that effect performance and emission are heat release, ignition delay, combustion phases, maximum heat release rate (HRRmax) in premixed phase, and combustion duration.25,42 More specifically emissions such as NOx, PM, THC, HC, CO formation and performance such as Break Specific Fuel Consumption (BSFC), Break Specific Energy Consumption (BSEC), and Brake Thermal Efficiency (BTE) are affected by combustion parameters. The effects of combustion on performance and emission varied with various parameters such as biodiesel feedstocks (sources), contents of biodiesel, cetane number, advance injection timing and combustion, oxygen content, engine load, engine speed, density and viscosity.13.1. Combustion chemistry and engine emission
Combustion is a complex phenomenon which creates heat and includes numerous free radical species and responsive intermediates in chain spreading, chain propagation and termination reactions. To understand the ignition and emission matters such as formation of pollutants, quantitative chemical information is necessary along with the nature and amount of undesirable and potentially noxious products.43 Combustion chemistry perspectives are included especially in ignition, heat release and elimination forms. They have a fundamental impact on harmful pollutant emission signatures with expansion in use of biofuels in diesel or biodiesel. For instance, particulate emission can be decreased by addition of ethanol44 however undesired aldehyde may be increased.45 Additionally an increased NOx emission is seen with increased biodiesel ignition.46 The biodiesel class of compounds is particularly diverse chemically, and to optimise burning methodology it is important to consider prototypical analysis.43Biodiesel fuels are methyl and ethyl esters of unsaturated fats from plant and animal provenance.47 Biodiesel is produced by artificially reacting lipids (e.g., vegetable oil, animal fat) with an alcohol to create unsaturated fatty esters. The unsaturated fat profile of biodiesel is similar to that of guardian oil or fat, which is a key variable that impacts its fuel qualities.48 Biodiesel can be mixed with petroleum diesel or utilized as a pure fuel, exploiting its low sulfur and aromatics content and of the considerable effect in diminishing in CO, unburned hydrocarbons, and particulates in the exhaust gases.49 Biodiesel fuel contains branched and unsaturated long carbon chains. Due to the larger size of these molecules and their chemical variability, the improvement of detailed combustion models is in its early stages.50
Several features of the combustion reactions for methyl esters such as methyl palmitate (C17H34O2), methyl stearate (C19H38O2), methyl oleate (C19H36O2), methyl linoleate (C19H34O2), and methyl linolenate (C19H32O2), are similar to those of large n-alkanes, such as n-hexadecane, because of the common alkyl chains.51 Combustion mechanisms have a tendency to become complex, not just due to the extent of the fuel molecules, but additionally as a result of the extra reactions of oxygen-containing species. For example, low-temperature ignition of methyl decanoate is characterized by 3012 species and 8820 reactions.52 Vital pathways for ester ignition incorporate alkyl peroxy radical reactions, isomerization reactions and H-atom exchange.53 Methyl esters can deteriorate into two different reactive oxygen carriers which could contribute to soot-precursor lessening, or the O–C–O structure in the molecule may lead to CO2.43 Ignition timing is truly diverse notwithstanding for a homologous arrangement of methyl esters, with methyl butanoate being the most safe fuel to auto-ignition. It was reported that the reactivity increases with the length of the alkane chain. High reactivity is attributed to the methoxy radical. More generally, alkyl and alkyl ester radicals add to O2 to form RO2 radicals in the low-temperature region, and they deteriorate in the high-temperature region to form olefins and unsaturated esters which may then react further in the same elementary reactions.54 The combustion reactions of ethyl esters show contrasts from those of methyl esters. Mole fractions for C2- to C6-hydrocarbons are higher in the ethyl formate flame as an outcome of the ethyl group and carbon development reactions. However, they are still lower than those seen in comparable fuel-rich hydrocarbon flames, affirming a propensity of ester fuels to lessen soot precursors. Concerning oxygenated intermediates, H-atom transfer from the methoxy group of the methyl ester drives especially to formaldehyde, and H-atom transfer from the ethoxy group of the ethyl ester prompts more prominent mole divisions of acetaldehyde.55 Ethyl esters can deteriorate through a unimolecular disposal reaction creating C2H4 by means of a six-membered pericyclic transition state. This ethene elimination reaction may be an explanation behind why ethyl esters may ignite more quickly.56
Fig. 3 demonstrates the destiny of the radical that is generated from methyl decanoate supply of an optional H-atom by OH radicals. A ROO radical is formed that isomerizes to three further distinctive radicals of QOOH structure at low ignition temperature of 650 K (Fig. 3a). The next stages in the oxidation include ketohydroperoxides and four- and five-membered cyclic ethers. During higher ignition temperature of 900 K (Fig. 3b), isomerization is seen to further C11 alkyl ester radicals. The reaction scheme also includes unsaturated compounds. As to decenoate oxidation, the double bond inhibits certain H-atom-transfer isomerization reactions. The vicinity and position of a double bond in this manner has an imperative impact on the low-temperature reactivity of the ester molecule.57 Isomeric mediums are essential for biofuel ignition as a result of extra chemical functional groups in the fuel molecules that offers pathways to distinct reaction sequences, and potential fuels might likewise have distinctive isomers.43 Blending of biofuels as additives in diesel biodiesel blends may spread more chemical pathways through the interaction of the decomposition and oxidation of all the compounds in the mixture. Hence different types of isomeric additives blends with biodiesel may lessen the existing problems of biodiesel fuelled engine.
 | ||
| Fig. 3 Flow decomposition paths of a radical formed by abstraction of a secondary H-atom from methyl decanoate at a residence time of 1 s and a temperature of (a) 650 K and (b) 900 K.57 | ||
3.2. Combustion and emission
At the primary phase of fuel injection, the cylinder pressure and temperature are a little lower with higher ignition delay and forms greater fuel rich zone with advanced SOI timing, which is responsible for increased CO emission. But at a constant SOI timing, CO emissions decreases with increasing fuel injection pressure.58 The retarded injection timing increases CO emission due to a longer heat release.59 The in-cylinder pressure and temperature drops with retarded SOI timing that increases the HC emission with increasing fuel injection pressure. With retarded SOI timing NOx emission decreased and reached to lowest when SOI timings is near TDC but begin to increase again when SOI timings were further retarded after TDC. Up to 4.125° CA ATDC SOI timing the peak of premixed heat release tends to increase but at 5.625° CA SOI timing the peaks of premixed heat release and NOx concentration reduced.58Lower gas pressure and in-cylinder temperature showed lowest NOx emission at 25% load.60 Decreasing maximum heat release rate (HRRmax) in the premixed combustion phase lowered the in-cylinder gas temperature and NOx emissions, but increased amount of fuel injected at full load increase the NOx emissions. Deterioration of atomization and combustion lowered the HRRmax and lengthen the premixed combustion duration at full load.61 Advanced SOI of B5 increase the NOx emission62 whereas B10 showed lowest NOx emissions at all loads, but due to its high viscosity, the smoke emissions increased by deteriorating the atomization and combustion.63,64 Shorter delay of CD of B5 leads to lower smoke emission and longer delay of CD of B10 leads to higher smoke emissions.65 The deterioration of combustion together with high fuel density caused a decrease in NOx.66,67 Higher cetane number of biodiesel causes a decrease in flame temperature and NOx.68,69 B5 showed shorter ignition delay than neat diesel and offered complete combustion with low total hydrocarbon (THC) emissions.68,70 However, THC emissions of B10 were higher than B5 and neat diesel at all loads due to larger spray droplet size owing to higher density and viscosity characteristics of the fuel, which deteriorates the combustion.71–73
Combustion temperature, the oxygen concentration and the duration of combustion is mainly responsible for NOx formation.74–76 Early start of combustion due to lower premixed burned fraction decreases the NOx emissions.63,69,76 At the initial stage of diffusion combustion higher amount of oxygen content causes the biodiesel to produce excess NOx with higher in-cylinder temperatures.77–79 Moreover, NOx formation rates in the post-flame gas region increased because of the longer existing time with the increase of the overall Combustion Duration.28,79 Combustion occurred earlier because of higher cetene number and lower aromatic content of biodiesel causes shorter ID period, which improved the THC emissions due to formation of over-lean regions. Also the longer CD with the increase in the engine loads due to higher boiling point of biodiesel also leads to improve the THC emissions.76,80–83 The higher rate of fuel flow with oxygen content and higher combustion temperatures are effective parameters with the increasing engine load to improve CO emissions.30,80,84–87 In addition, early start of combustion and longer combustion duration increases the possibility of CO oxidation to CO2 with biodiesel addition for the higher engine loads.65,80,84,88–91 Presence of oxygen content in the biodiesel leads to more clean and complete combustion which reduce the CO and HC emissions. Advanced injection timing with biodiesel reduce CO and HC emissions but increases the NOx formation due to increases the fuel existence time in pump-line-nozzle injection system.27
3.3. Combustion and performance
Delayed combustion duration (CD) has negative effects on the engine performance.92–94 Increasing percentages of biodiesel in the blends showed early SOC which increases the pumping work and heat loss and increase the combustion duration, causing heat release in the expansion stroke which contributes less work.90 Retarded start of injection timing showed minimum break specific fuel consumption (BSFC) with increasing fuel injection pressure. But at a constant start of injection timing thermal efficiency increases with increasing fuel injection pressure.58 Break thermal efficiency (BTE) increased with increasing fuel injection pressure at full load for biodiesel.95 Lower in-cylinder pressure and temperature increases the Break Specific Energy Consumption (BSEC) of increasing proportion of biodiesel–diesel in blends at low load, because of biodiesel having higher injection duration and combustion duration. Increasing in BSEC in-turn lowered the brake thermal efficiency (BTE) than neat diesel. Poor atomization and mixture formation is attributed to slow down the combustion and lower BTE.273.4. Impact of additives on performance and emission
A lot of research have been performed on additives in diesel, biodiesel and their blends. Rashedul et al.96 reviewed the performance and emission of biodiesel with additives in diesel engine and reported that oxygenated additives in blends is not efficient to improve brake power and less efficient to decrease fuel consumption, while antioxidant additives and metal-based additives improves the brake power, and decrease fuel consumption. On the other hand, Oxygenated additives, metal-based additives and antioxidant additives showed reduced NOx emission. Metal-based additives, ethanol and methanol decreased the CO and HC. Smoke opacity of blend fuels with additives also diminished with DEE, ethanol and metal-based additives. Misra et al.97 reported that ethanol as an additive is more significant to improve combustion performance and emission. The National Renewable Energy Laboratory’s (NREL) report “NOx Solutions for Biodiesel” reported that di-tert-butyl peroxide and ethylhexyl nitrate, which are both cetane improvers effectively reduce NOx emission. NREL also reports that tert-butyl hydroquinone, an antioxidant, is an effective NOx reducing agent, with slight PM increase.25 Kannan et al.98 showed that, using FeCl3 as fuel-borne catalyst to diesel engine increases BTE by 6.3% due to decreased fuel consumption by 8.6%. Including 1% of 4-nonylphenoxyacetic acid additive increases BTE and decreased the exhaust emissions.99 Keskin et al.100 mentioned that specific fuel consumption of tall oil with metallic additives showed decreasing trends than biodiesel. Chen et al.101 illustrated that emulsified bio-solution/palm-biodiesel/diesel blends showed benefits to save energy and decreases the polycyclic aromatic hydrocarbons and particulate matter. Usually, catalysts do not affect the engine performance significantly but delay the ignition time and reduce unburned hydrocarbons (HC) and particulate matter (PM). However, overall performance can be enhanced by adding additives with the fuel.1024. Fuel additives
4.1. Introduction to fuel additives
Fuel additives are natural substances dissolvable in fuels. Around 20 properties of fuels can be enhanced, retained or bestowed new advantageous attributes by the inclusion of small amounts of specific chemicals denoted as fuel additives. Fuel additives are included at a level from a few ppm to a few thousand ppm. It is imperative that additives which improve some properties do not impair different other properties and fuel quality. Some of these additives may help to maintain fuel quality (e.g., antioxidants, stabilizers, corrosion inhibitors, and biocides). Others may help the development of fuel through the dispersion into the vehicle tank (e.g., flow improvers, pipeline drag reducers, demulsifiers and antifoams); may be included for legal reasons (e.g., colors and markers) or can address particular concerns from engine manufactures (e.g., deposit control additives and lubricity improvers).103 Fuel additives in diesel, biodiesel and their blends improves the fuel characteristics of hence show the following benefits:24,96• Suppression of corrosion of fuel tanks, channel lines etc.
• Suppression of catastrophic wear of fuel system equipment in the diesel engine.
• Diminished pumping expenses and energy use in long-distance fuel pipelines.
• Improvement in diesel cetane, octane parameters.
• Improvement of cold flow in middle distillates, boosting utilization of biofuel.
• Changes of stability to enhance long time storage of fuels.
• Improved vehicle performance and economy.
• Decrease in noxious emissions.
• Enhanced fluid stability over a more extensive range of conditions.
• Improvement of viscosity number and reduction of the rate of change of viscosity with temperature.
• Enhanced ignition by decreasing delay time, flash point, etc.
• Reduction of wear with agents that adsorb onto metal surfaces and provide chemical to-chemical contact as opposed to metal-to-metal contact under high-load conditions.
However, as fuel additives comprise of several chemicals, some of them are harmful for the environment. Then there are certain bio-elements within additives which can cause potential harm to the engine if not used properly.104 Higher proportion of alcohol causes extra release of rust, debris, sediment and gunk and further clogging and damage to engine components and filters.105 For instance, it is very difficult to use ethanol fuel in cold weather.106 Higher concentration of antioxidants showed a remarkable increase of acid values at antioxidant levels of 1000 mg kg−1.107
4.2. Application of various types of fuel additives in diesel engine
The reaction path diagram of combustion of some fuel additives are given below. Fig. 4a–d show the reaction path diagram of (a) ethanol, (b) methanol, (c) n-butanol and (d) cerium oxide combustion and Fig. 4e shows the oxidation process of antioxidants.
 | ||
| Fig. 4 (a) Reaction path diagram for ethanol combustion.152 (b) Reaction path diagram for methanol combustion.152 (c) Reaction path diagrams for high-temperature combustion of n-butanol.152 (d) Cerium oxide role in combustion process: it absorbs oxygen from NO mediates produced due to the high temperature of combustion chamber, then donates this oxygen to the soot (C) particles produced by incomplete combustion of hydrocarbons and converts them to CO2 molecules.16 (e) Antioxidants form stable radical intermediates with moderate resonance delocalization, which hinders the oxidation of fuels.153 | ||
5. Fuel properties, fatty acid composition and engine combustion
5.1. Fuel properties and engine combustion
The fuel properties of biodiesel will influence the engine performance and emissions followed by combustion, since it has distinctive physical and chemical properties from petroleum-based diesel fuel. Biodiesel contains about 10–15% of oxygen by weight154 and has higher cetane number, higher viscosity and higher specific gravity that influences fuel quantity, injection timing and spray characteristics. Combustion is also influenced by fuel viscosity.19 Biodiesel has lower heating value of almost 12% than diesel fuel that will reduce power. Although increase in amount of fuel injected increases the heating value, injection duration increase due to change in start of injection will affect the ignition delay. However, higher cetane number of biodiesel decreases the ignition delay and advances the combustion timing.155 The lower compressibility of biodiesel and its blends facilitates quick pressurization of the injected fuel into the pump that can accelerate the pressure wave towards the injectors to advance the injection timing. The higher density of biodiesel is also responsible to advance the injection timing. Moreover, the higher viscosity increases the injection line pressure with lower vapor content in a high pressure injection system, and advances the injection timing which leads to a decrease in ignition delay.25,73The fuel-bound oxygen in the blends is a fundamentally common property and is a parameter that impacts the burning characteristics by means of affecting the local fuel–air proportion in the different zones and thus the temperature and emission formation. This was proved very effective. The remaining critical properties of the bio-fuel blends, mainly the bulk modulus of the elasticity, kinematic viscosity, lower heating value and latent heat of evaporation, accomplish different values for the same value of fuel-bound oxygen in the mixer. Vegetable oil blends increase the kinematic viscosity that decreases the fuel evaporation rate and give poor mixing behavior, which leads to a monotonic increase in the soot emissions. On the other hand, the low kinematic viscosity of DEE may have again brought about far from ideal fuel evaporation and mixing in the spray, as confirmed by the delayed lower pressures and temperatures, which did not have any unfavorable impacts on the soot and NOx emissions or the brake thermal efficiency. The calorific value of the bio-fuel blends has an expected increment on the brake specific fuel consumption due to the increase in fuel-bound oxygen in the blends. Hence, for the biofuels that have lower calorific values, the fuel requirement increases for the same power output. The higher latent heat of evaporation, as in the case of bio-alcohols and DEE, causes lower temperatures during evaporation and thus higher ignition delays and lower gas temperatures after combustion. Lower cetane index of bio-alcohols and DEE blends with diesel fuel showed longer ignition delay and displacement-delay of heat release rate, whereas bio-diesel and vegetable oil cause lower ignition delays and displacement-advance of the HRR diagram was found. Furthermore, there is an interchange of the dissipated fuel–air and temperature in the different “zones” relating to the heat released by the combusted measure of fuel, as affected by the ignition attributes, latent heat of evaporation, lower heating value (LHV), cetane number and so on, which determine the final burning temperature and oxygen lack or excess.156
However, introducing additives in diesel, biodiesel and their blends improves many of the major properties, and hence improves combustion. Rashedul et al.96 concluded that addition of metal-based additives improve the flash point, decrease the pour point and viscosity of biodiesel while oxygenated additives lessen the density and viscosity and increase the oxygen content of biodiesel. Higher combustion efficiency results from higher oxygen content, lower density and viscosity of oxygenated additives.18 The addition of antioxidants improve flash point, cetane number and oxidation stability but decreases the calorific value of biodiesel.
Table 5 shows the changes in values of various physico-chemical properties such as density, viscosity, flash point, fire point, cetane index, oxygen content, sulfur content, latent heat of evaporation etc. of different proportions of diesel, biodiesel and their blends with and without additives.
| Biodiesel feedstock | % Additive | Fuel properties | Ref. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density/g cm−3 (20 °C) | Viscosity/Pa s (40 °C) | Flash point/°C | Fire point/°C | Pour point/°C | Cetane number | Calorific value/MJ kg−1 | %O | %S | %C | %H | ΔHevap/kJ kg−1 | ||||
| a Density at 25 °C.b Density at 15 °C.c Viscosity at 20 °C. | |||||||||||||||
| Diesel (C12H23) | — | 0.82 | 3.4 | 71 | 1 | 45 | 43.2 | 13.4 | <10 | 87.4 | — | — | 96 | ||
| Diesel | Ethanol (C2H5H) | 5% | 0.833a | 4.05 | 58.6 | 72.4 | — | 45.53 | 44.09 | 1.8 | — | 84.8 | 13.4 | — | 157 |
| Diesel | 2,5-DMF | 30% | 0.85 | 2.32c | — | — | — | — | 39.73 | 5.27 | — | 83.2 | 11.53 | — | 158 |
| Ultra low sulfur diesel (ULSD) | — | 0.840 | 2.4 | — | — | — | 52 | 42.5 | 0 | <10 | 86.6 | 13.4 | 250–290 | 159 | |
| Waste cooking oil | — | 0.871 | 4.6 | — | — | — | 51 | 37.5 | 10.8 | <10 | 77.1 | 12.1 | 300 | 159 | |
| Waste cooking palm oil | FeCl3 as a Fuel Borne Catalyst (FBC) | 5 μmol | 0.8658 | 4.55 | 170 | 190 | 9 | 67.4 | 38.1 | — | — | — | — | — | 98 |
| 15 μmol | 0.8652 | 4.52 | 167 | 186 | 9 | 68.1 | 38.21 | — | — | — | — | — | |||
| 25 μmol | 0.8646 | 4.54 | 167 | 185 | 9 | 68.9 | 38.32 | — | — | — | — | — | |||
| 35 μmol | 0.8652 | 4.56 | 166 | 184 | 9 | 69.2 | 38.28 | — | — | — | — | — | |||
| 50 μmol | 0.8658 | 4.57 | 165 | 183 | 9 | 69.6 | 38.43 | — | — | — | — | — | |||
| Jatropha biodiesel and diesel | Ethanol | 20% | 0.832b | 2.38 | 14 | — | −3 | 50 | 39.93 | 7.77 | — | 78.69 | 13.54 | — | 160 |
| 30% | 0.834b | 2.4 | 12.5 | — | −9 | 50 | 38.96 | 12.21 | — | 74.49 | 13.30 | — | |||
| 40% | 0.820b | 2.018 | 12 | — | −12 | 41 | 36.33 | 14.53 | — | 72.07 | 13.41 | — | |||
| Waste cooking oil and (ULSD) | Ethanol | 5% | 0.842 | — | — | — | — | — | 41 | 3.3 | — | — | — | — | 159 |
| 10% | 0.839 | — | — | — | — | — | 40.3 | 5 | — | — | — | — | |||
| 20% | 0.833 | — | — | — | — | — | 38.9 | 8.2 | — | — | — | — | |||
| RME | — | — | 0.884 | 4.79 | 178 | — | — | 53.4 | — | 10.9 | — | — | — | — | 161 |
| RME–diesel blends | Ethanol | 10% | 0.844 | — | — | — | — | — | 40.75 | 3.9 | — | — | — | — | 44 |
| 20% | 0.84 | — | — | — | — | — | 39.03 | 7.79 | — | — | — | — | |||
| 30% | 0.834 | — | — | — | — | — | 37.54 | 11.1 | — | — | — | — | |||
| Soybean biodiesel | — | 0.865 | 4.78 | — | — | — | 49 | 41.20 | — | — | — | — | — | 162 | |
| Soybean biodiesel and diesel | Ethanol (4%), Isopropanol (1%), alumina (100 mg) | 0.840 | 3.37 | — | — | — | 52 | 42.59 | — | — | — | — | — | ||
| Soybean biodiesel and diesel | Ethanol | 5% | 0.840 | — | — | — | — | — | 40.94 | — | — | — | — | 266 | 163 |
| Diethyl ether (C2H5)2O | 0.837 | — | — | — | — | — | 41.41 | — | — | — | — | 242 | |||
| Soybean biodiesel and diesel | Methanol (CH4O) | 5% | 0.842 | — | — | — | — | — | 39.68 | 7.13 | — | — | — | 264 | 164 |
| 10% | 0.843 | — | — | — | — | — | 38.84 | 9.08 | — | — | — | 294 | |||
| Jatropha biodiesel | — | 0.861 | 4.27 | 202.5 | 2 | 53.5 | 39.83 | — | — | — | — | — | 165 | ||
| Jatropha biodiesel | Ethanol | 5% | 0.875a | 5.29 | 147.9 | 167.4 | — | 48.85 | 41.24 | 12.2 | — | 76 | 11.8 | — | 157 |
| Jatropha biodiesel and diesel | Ethanol | 5% | 0.842a | 4.31 | 77.4 | 92.4 | — | 46.23 | 43.49 | 4 | — | 82.9 | 13.1 | — | 157 |
| Jatropha biodiesel and diesel | n-Butanol (C4H10O) | 5% | 0.834 | 3.29 | 87.5 | — | — | — | 43.40 | — | — | — | — | — | 165 |
| 10% | 0.831 | 3.24 | 79.5 | — | — | — | 43.15 | — | — | — | — | — | |||
| Diethyl ether | 5% | 0.830 | 3.22 | 83.5 | — | — | — | 43.39 | — | — | — | — | — | ||
| 10% | 0.823 | 3.15 | 71.5 | — | — | — | 43.10 | — | — | — | — | — | |||
| Cotton seed oil biodiesel | — | 0.871 | 5.28 | — | — | — | 51 | 37.5 | 10.8 | <10 mg kg−1 | 77.1 | 12.1 | 300 | 166 | |
| Cotton seed oil biodiesel and diesel | Ethanol | 5% | 0.867 | — | — | — | — | — | 37.1 | 11.9 | — | 76 | 12.1 | 324.5 | |
| 10% | 0.862 | — | — | — | — | — | 36.7 | 13 | — | 74.8 | 12.2 | 349.2 | |||
| 15% | 0.852 | — | — | — | — | — | 36.2 | 14.1 | — | 73.7 | 12.2 | 374.2 | |||
| B20 biodiesel | Methanol (5% by vol.) | 0.843 | 3.1345 | 43 | — | — | 92.4 | — | — | — | — | — | — | 167 | |
| Neem oil biodiesel | — | 0.867 | 4.5 | 165 | — | — | 51 | 41 | 11 | — | — | — | — | 20 | |
| Neem oil | Diethyl ether | 5% | 0.859 | 4.2 | 148 | — | — | 54 | 40.6 | 11.44 | — | — | — | — | 20 |
| 10% | 0.851 | 3.89 | 127 | — | — | 58 | 40.3 | 11.88 | — | — | — | — | |||
| 15% | 0.844 | 3.57 | 102 | — | — | 61 | 40.0 | 11.26 | — | — | — | — | |||
| Kapok biodiesel | — | 0.850 | 4.1 | 105 | — | −8 | 52 | 41.09 | — | <0.0005 | — | — | — | 168 | |
| Kapok biodiesel and diesel | 1,4-Dioxane (C4H8O2) | 0.5% | 0.853 | 3.76 | 95 | — | −10 | 54 | 41.01 | — | <0.0005 | — | — | — | |
| 1% | 0.851 | 3.65 | 86 | — | −14 | 56 | 40.92 | — | <0.0005 | — | — | — | |||
| Karanja biodiesel | — | 0.889 | 5.71 | 181 | — | — | 52.8 | 39.13 | 10.8 | — | — | — | — | 169 | |
5.2. Impact of fatty acid composition on fuel properties and engine combustion
Pinzi et al.64 studied the impact of fatty acid composition of biodiesel on combustion behavior in a diesel engine. The authors concluded that the viscosity of vegetable oil raises with the carbon chain length, while the lubricity increases slightly. However, enhancing the degree of unsaturation resulted in lower viscosity, but there is no significant change in lubricity. The calorific value of the fuel also depends on chain length and as the degree of unsaturation rises the calorific value reduces.170 Mehta et al.171 have also drawn a similar conclusion about the effect of carbon chain length and degree of unsaturation on the calorific value of the fuel. The CN of the fuel enhances with carbon chain length and reduces with the degree of unsaturation.172,173 The adiabatic flame temperature also varies with carbon chain length and degree of unsaturation. An increasing adiabatic flame temperature implies an increase in neighboring burning temperature in the ignition chamber and leads to generate large amount of NOx.47,174 Table 6 details the fatty acid composition of different vegetable oils.| Fatty acid | Palm | Olive | Peanut | Rape seed | WCO177 | Soya bean | Neem oil178 | Cotton seed179 | Jatropha curcas L.178 | Sunflower | Karanja178 | Grape | H.O. sunflower | Almond | Corn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lauric | C12:0 | 0.1 | 0 | 0 | 0 | — | 0 | — | — | — | 0 | — | 0 | 0 | 0 | 0 |
| Myristic | C14:0 | 0.7 | 0 | 0.1 | 0 | — | 0 | 0.2–0.26 | — | 1.4 | 0 | — | 0.1 | 0 | 0 | 0 |
| Palmitic | C16:0 | 36.7 | 11.6 | 8 | 4.9 | 38.8 | 11.3 | 14.9 | 24.3 | 12.7 | 6.2 | 3.7–7.9 | 6.9 | 4.6 | 10.4 | 6.5 |
| Palmitoleic | C16:1 | 0.1 | 1 | 0 | 0 | — | 0.1 | 0.1 | — | 0.7 | 0.1 | — | 0.1 | 0.1 | 0.5 | 0.6 |
| Stearic | C18:0 | 6.6 | 3.1 | 1.8 | 1.6 | 4.1 | 3.6 | 20.6 | 2.2 | 5.5 | 3.7 | 2.4–8.9 | 4 | 3.4 | 2.9 | 1.4 |
| Oleic | C18:1 | 46.1 | 75 | 53.3 | 33 | 47.9 | 24.9 | 43.9 | 16.4 | 39.1 | 25.2 | 44.5–71.3 | 19 | 62.8 | 77.1 | 65.6 |
| Linoleic | C18:2 | 8.6 | 7.8 | 28.4 | 20.4 | 0.2 | 53 | 17.9 | 54.9 | 41.6 | 63.1 | 10.8–18.3 | 69.1 | 27.5 | 7.6 | 25.2 |
| Linolenic | C18:3 | 0.3 | 0.6 | 0.3 | 7.9 | — | 6.1 | 0.4 | 0.1 | 0.2 | 0.2 | — | 0.3 | 0.1 | 0.8 | 0.1 |
| Arachidic | C20:0 | 0.4 | 0.3 | 0.9 | 0 | — | 0.3 | 1.6 | — | 0.2 | 0.3 | 4.1 | 0.3 | 0.3 | 0.3 | 0.1 |
| Gadoleic | C20:1 | 0.2 | 0 | 2.4 | 9.3 | — | 0.3 | — | — | — | 0.2 | 2.4 | 0 | 0 | 0 | 0.1 |
| Behenic | C22:0 | 0.1 | 0.1 | 3 | 0 | — | 0 | — | — | — | 0.7 | — | 0 | 0.7 | 0.1 | 0 |
| Erucic | C22:1 | 0 | 0 | 0 | 23 | — | 0.3 | — | — | — | 0.1 | — | 0 | 0 | 0 | 0.1 |
| Lignoceric | C24:0 | 0.1 | 0.5 | 1.8 | 0 | — | 0.1 | — | — | — | 0.2 | — | 0 | 0.3 | 0.2 | 0.1 |
| Nervonic | C24:1 | 0 | 0 | 0 | 0 | — | 0 | — | — | — | 0 | — | 0 | 0 | 0.4 | 0 |
| Saturated180 | — | 49.3 | 14 | 16.9 | 7.365 | — | 15.65 | — | 25.9 | — | 10.1 | — | — | — | — | 12.948 |
| Monounsaturated180 | — | 37 | 72 | 46.2 | 63.276 | — | 22.783 | — | 17.8 | — | 45.4 | — | — | — | — | 27.576 |
| Polyunsaturated180 | — | 9.3 | 14 | 32 | 28.142 | — | 57.74 | — | 51.9 | — | 40.1 | — | — | — | — | 54.677 |
Hellier et al.175 investigated the effects of fatty acid composition of biodiesel on diesel engine combustion characteristics. It was reported that, the majority of the vegetable oils showed a range of ignition delay between ±0.6 CAD than that shown by base diesel, however, greatly lessened rates of peak heat release rate were found. Whereas, with an increase in C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H ratio (carbon to hydrogen ratio) an increased ignition delay was observed. The HRRpeak reduced with reducing the viscosity of vegetable oils. The peak pressures raises as the degree of unsaturation increases, i.e. in case of large unsaturated fatty acid (C18:2). It was found that increasing degree of unsaturation advanced the SOC because of increasing CN and bulk modulus which in turn reduced the ignition delay. As the chain length increases, the initial cylinder pressure increased to a small extent.64
H ratio (carbon to hydrogen ratio) an increased ignition delay was observed. The HRRpeak reduced with reducing the viscosity of vegetable oils. The peak pressures raises as the degree of unsaturation increases, i.e. in case of large unsaturated fatty acid (C18:2). It was found that increasing degree of unsaturation advanced the SOC because of increasing CN and bulk modulus which in turn reduced the ignition delay. As the chain length increases, the initial cylinder pressure increased to a small extent.64
6. Effect of additives on combustion behaviour
An et al.181 investigated the combustion characteristics of waste cooking oil biodiesel with ethanol as additives. Simulations were performed for each blend with or without advanced injection timing of 2°, 3° and 5° crank angle for each load. They reported that increasing percentage of ethanol in the blend with no injection advance, decreased the peak cylinder pressure at light load (10% loads) by 15.3%, 25.1% and 40.0% for 5%, 10% and 20% ethanol, respectively. Chen et al.44 studied the combustion characteristics of rapeseed oil on addition of ethanol as fuel additive and found that both 20% and 30% ethanol in biodiesel lengthen the ignition 1.2 CA and 2.5 CA compared to diesel, respectively. The HRRmax with ethanol blended fuel was found to be higher, because of prolonged ignition delay and faster rate of evaporation of ethanol in the premixed combustion phase, which also leads to increase the peak pressure in the cylinder.182–184 The ethanol also shortens combustion duration in the order of 69.6° CA, 54° CA and 48° CA for E0, E10 and E30, respectively. Kannan et al.157 investigated the impact of ethanol addition to jatropha methyl ester through port injection on combustion characteristics. They found that, the maximum in-cylinder peak pressure for 5% ethanol with neat diesel and diesel–jatropha blends were found at about 67 bar and 66 bar, respectively. Ethanol in diesel showed an increase in pressure of almost −5° to −1° (BTDC) with delayed maximum peak pressure of 8° (ATDC) than that of neat diesel and JME. Results showed a slow rise of pressure from −10° to −2° (BTDC) at premixed stage but at diffusion stage the maximum peak pressure reached at about 15° (ATDC) which expanded up to 50° (ATDC) successively. They also reported in this case that 25% of biofuels addition may show a nearest ignition behavior to that of diesel. Anbarasu et al.166 studied the effect of ethanol in cotton seed oil biodiesel operated diesel engine on combustion. It was observed that, the heat release rate of BE blends decreases from 99 kJ per m3 per degree to 61 kJ per m3 per degree for diesel and BEB15, respectively. It was found that the maximum cylinder pressure decreases from 71.5 bar to 70 bar for the blends of BEB5 and BEB 15, respectively. This is due to the cooling effect of the higher blends of ethanol. Increased ethanol percentage in the blend retarded the start of combustion and decreased the combustion duration.159 Hulwan et al.160 investigated the combustion behavior of jatropha biodiesel–diesel–ethanol blends with high ethanol contents of 20%, 30% and 40% with advanced injection timing. The observation showed that, adding high proportion ethanol content led to higher cylinder pressure but the peak cylinder pressure occurs later at low loads. Results demonstrated an ID increment around 40–50% at 1600 rpm with 21° injection timing. Labeckas et al.161 studied the effect of ethanol and diesel–biodiesel (rapeseed methyl ester) blends on combustion properties at different air fuel mixtures. Results demonstrated that the auto-ignition delay of E15B was 15.4% higher compared to standard diesel at richer air–fuel mixture of 1.5 at engine speed of 2200 rpm. It was also reported that the increase of auto-ignition delay was 43.4%, 18.9%, 14.0% for overall lean, 21.1%, 22.6%, 22.4% for moderate and 14.9%, 21.3%, 15.4% for richer air–fuel mixtures at 1400, 1800 and 2200 rpm speeds for E15B blend. The angle of average maximum heat release rate moved from −0.8° BTDC (DF) to +2.0° ATDC (E15B) CADs with increased fuel mass from 0.4 wt% to 6.1 wt% for lean air–fuel mixture of λ = 5.5 at low speed of 1400 rpm. The maximum pressure in the cylinder increased by 2.1 bar (3.1%) and 1.2 bar (1.9%) when running E15B blends against values of 68.1 bar and 64.6 bar of neat diesel operating on richer air–fuel mixtures λ = 1.5 at 1400 and 2200 rpm speeds. There was also a rise in maximum in-cylinder pressure of 1.65 bar per degree and 0.61 bar per degree higher than ordinary diesel running on richer air–fuel mixtures λ = 1.5 at 1400 and 2200 rpm speeds, respectively. The maximum heat release rate increased in the range of 114.7 to 146.7 kJ per m3 per degree (27.9%) and 93.9 (DF) to 111.3 (E15B) kJ per m3 per degree (18.5%) for richer mixtures of 1.5 at 1400 and 1800 rpm speeds.Shaafi et al.162 studied the combustion characteristics using alumina as a nano-additive with two modified fuel blends, B20 (diesel–soybean biodiesel) and diesel–soybean biodiesel–ethanol blends. Results showed that adding alumina with diesel–soybean biodiesel–ethanol blends increased the ignition delay at lower loads but as load increased the ignition delay decreases compared to neat diesel due to increased temperature. A sharp increasing in the cylinder pressure was observed up to 7° ATDC from 7° BTDC in the case of adding alumina to the fuel blend. The peak pressure for alumina added fuel blend at all the loads increased to 64.61 bar, whereas for neat diesel and B20 blend, the values were 63.03 bar and 62.41 bar, respectively. Addition of alumina nanoparticles in the blends give higher heat release as 62 J/CA, compared to values of 51.60 J/CA, 53.12 J/CA for neat diesel and B20 blend, respectively. In spite of lower calorific value of alumina added fuel blend, it has higher instantaneous HRR between 6.5° CA and 1° BTDC that enhances the combustion rate and leads to complete combustion. Kannan et al.98 investigated the influences of metal-based additives on combustion characteristics of ferric chloride (FeCl3) as a fuel-borne catalyst (FBC) blended to waste cooking palm oil-based biodiesel. It was concluded that a slightly higher maximum cylinder gas pressure (CGPmax) of 75.8 bar and 366.1° CA with FBC added biodiesel and 74.3 bar and 367.2° CA without FBC added biodiesel was found, respectively, at optimized operating condition. Advanced injection timing and increased injection pressure was attributed to this as they perform earlier SOC and shorter ID of biodiesel. FBC added biodiesel showed a HRRmax of 29.2 J per degree CA, which is 4.6% higher than that of biodiesel without FBC and 6.2% higher than that of diesel at optimized operating condition. Higher amount of fuel accumulated and injected earlier at the compression stroke due to lower ignition delay was responsible for this.185 A reduction in ignition delay was found with the increase in injection pressure from 220 to 280 bar due to better atomization and efficient mixing of fuel with air,186 but accelerating the injection timing from 23° BTDC to 25.5° BTDC increases the ignition delay. FBC in biodiesel showed lower ignition delay of 10° CA, which is slightly lower than that of biodiesel without FBC at optimized operating condition. Addition of FBC in biodiesel showed lowest combustion duration of 52.8° CA at optimized operating condition. Improved combustion rate in premixed and controlled combustion phase leads to higher gas temperature and reduced CD at higher BMEPs. Selvan et al.112 investigated the effect of cerium oxide nanoparticles (CON) and carbon nanotubes (CNT) as fuel-borne additives in diesterol blends on combustion. The main findings of the experimental analysis showed that the peak pressure for the E20-CON 50-CNT 50 blends was found as 10.7 MPa at CA of 367°, whereas for the E20 blend it is 7.9 MPa at CA of 377°. Introducing CON provides oxygen and CNT prompts the combustion in the diesterol blend which increases the peak pressure and causes increase of cylinder gas pressure. The blending of CON and CNT reduced the ignition delay and leads to earlier start of combustion, this resulted in lower heat release rate and advanced peak heat release rate was found. The HRRmax was observed as 67 J/CA at a crank angle of 370° for the E20-CON 25 and CNT 25 blend, whereas it is 85 J/CA at a crank angle of 376° for the E20 blend.
Imtenan et al.165 studied the combustion behaviour of a diesel engine fuelled with diesel–jatropha biodiesel blend with 5–10 vol% n-butanol and diethyl ether. It was found that modified blends of J20 with n-butanol additives showed maximum in-cylinder pressure of 8–10.5° CA ATDC and increased accordingly with increasing speed. At 3000 rpm, the maximum in cylinder pressure of 86.95 bar and 86.07 bar was observed for J15B5 and J10B10 blends at 9.4° ATDC and 9.9° ATDC, respectively. Decreasing pressures with the increasing of the percentage of n-butanol is attributed to the lower calorific value of n-butanol compared to diesel and biodiesels.187 Retarded SOC of J15B5 was observed on −3.9° ATDC and for J10B10 it was −3.5° ATDC, whereas for J15D5 and J10D10 it was found at −3.7° ATDC and −3.1° ATDC on average for 1000, 2000 and 3000 rpm speeds, respectively. Lower cetane number leads to SOC later with prolonged ignition delay.188,189 HRR at premixed combustion decreases for n-butanol additives, however, at diffusion phase HRR was improved for additive blend compared to neat J20. They also reported that maximum in-cylinder pressures for J15D5 and J10D10 were 86.92 and 86.10 bar, respectively, at 10.1° ATDC and 10.4° ATDC at 3000 rpm engine speed. Although DEE has larger cetane index, SOCs of DEE blends were delayed because of higher latent heat of evaporation,190 which also lowered the maximum in-cylinder pressures and the peak pressures for DEE blends, as combustion occurred in a lower temperature environment. It was also revealed that 10% blends of the additives showed more delayed SOCs than 5% blends. HRR for DEE showed similar characteristics as n-butanol. Similar results were concluded by Imtenan et al.191 for 15% of palm biodiesel with 5% ethanol, n-butanol and diethyl ether additives. Sukjit et al.192 studied the effect of addition of ethanol and butanol as additives in RME and found that using EGR retarded the start of combustion as less air was used in the combustion process.
Hou et al.193 studied the combustion characteristics of a turbocharged compression ignition engine fuelled with dimethyl ether with used frying oil as biodiesel in the blends. It was found the peak heat-release rate of biodiesel, DME50, DME70 and DME100 were 241.9 J per degree CA at 11.5° CA ATDC, 210.7 J per degree CA at 12.5° CA ATDC, 208.8 J per degree CA at 17° CA ATDC and 186.9 J per degree CA at 19° CA ATDC, respectively. They reported that the peak in-cylinder temperature for biodiesel, DME50 DME70 and DME100 is 2097 K, 1954 K, 1914 K and 1838 K with retarded phase of 26° CA, 30.5° CA, 32.5° CA and 39.5° CA.
Qi et al.164 evaluated the effects of blending methanol as additive to soybean biodiesel–diesel blends on combustion characteristics. They observed that, at low engine load (BMEP = 0.177 MPa), both the blends showed similar peak cylinder pressure and peak ROPR but higher peak HRR than that of BD50 with low engine speed of 1500 rpm. However, as the engine speed increased to 1800 rpm, the peak cylinder pressure and peak ROPR of additives blended fuels are lower but the HRRpeak was similar to that of BD50. At high engine load (BMEP = 0.531 MPa), 5% methanol blends showed slightly higher HRRpeak than 10% methanol blends and BD50 at engine speed of 1500 rpm whereas during 1800 rpm, it is almost identical for all fuels. HRRpeak for BD50 showed earlier than methanol added blends. While Anand et al.169 reported that 10% of methanol in neat karanja oil (100%) reduced the cylinder pressure, HRR with increased ID. Lower cetane number and higher viscosity is responsible for this. Yasin et al.167 analyzed the combustion characteristics of diesel engine adding 5% methanol as fuel additive with B20 fuel blends. The main findings of the experimental work showed higher ROPR for B20 M5 than normal diesel. Higher mass of oxygen content of B20 M5 decreases the ignition timing with an earlier CD and higher maximum cylinder pressure. The maximum rate of heat release for B20 M5 was found as 363.1 J per degree CA at 13 CAD with B20 while for mineral diesel it was 358.7 J per degree CA at 14 CAD. Higher mass of oxygen content in B20 M5 notably improved the diffusion combustion zone and reduced the combustion duration by 7.1% relative to normal diesel. The start of combustion for B20M5 was found to be delayed. Higher fuel consumption rate and maximum pressure may attributed to this behavior.194
Lü et al.128 evaluated the combustion phenomenon of ethanol (5%, 10%, 15% and 20% ethanol), DMC (10, 20 and 30%), and DMM (10 and 20%) mixed with diesel. Results concluded that the maximum heat release rate (HRRmax) reduced with the increased percentage of ethanol and DMC at low engine loads while increased with increase of DMC and ethanol in blends at high loads. The ignition delay of all kinds of fuel blend was found delayed at increased levels of ethanol, DMC and DMM volumes at lower engine loads with oxygenated fuels in excess of 15% by volume. The combustion duration decreased with the increase of ethanol, DMC and DMM additives in diesel blend fuels at the same engine load. This is mainly due to increase the ignition delay, which leads to more homogeneous air–fuel mixtures and faster burning at premixed combustion stage.
Vallinayagam et al.138 investigated the impact of 1.5% IAN (isoamyl nitrate) and 1.5% DTBP (di-tert-butyl peroxide) as ignition promoting additives on diesel engine combustion characteristics fuelled by pine oil–diesel blend. The fuel burning rate in premixed combustion stage reduced to 34.5% and 33.1% from 40.4% of diesel–biodiesel blends for both additives, respectively. The ignition delay was reduced by 2° CA for IAN and 3° CA for DTBP compared to diesel–biodiesel blends. This is because IAN (C5H11NO2) added to the pine oil (C10H18O + C10H16) and diesel (C14H28) generates free alkoxy radicals whereas DTBP in same blends generates two alkoxy radicals, hence improving the ignition attributes.195
Chen et al.158 studied the combustion behavior of a diesel engine fuelled with 30% by volume of 2,5-dimethylfuran, n-butanol and gasoline with diesel. The main findings of the experimental results showed that, D30 has prolonged ignition delay due to lower cetane index, that leads to increase the pressure rise rate with quicker mass burning rate. Heat transfer losses also decrease with retarded CA50 as the ROPRmax decreased with this. D30 showed the shortest combustion duration and bulk mean gas temperature compared to B30, G30 and neat diesel. The ROPRmax of D30 is obviously higher than that of diesel fuel, which will exceed 10 bar per degree CA when CA 50 is advanced beyond 6° CA ATDC, which leads to create higher combustion noise and mechanical load. Increase of EGR rate also lengthens the ignition delay relative to B30, G30 and neat diesel.
Vedharaj et al.168 studied the effect of 1,4-dioxane with kapok biodiesel on combustion characteristics of diesel engine. It was concluded that the ignition quality of fuel blends increased with the increase of additives that lowered the ignition delay. The amount of fuel combusted in the premixed combustion stage was reduced to 24.86% for B25-10 ml from 31.2% for B25. Early start of combustion was found while additives introduced in biodiesel restrict the mixture accumulation and reduced the peak heat release rate.
Details of the effects of additives on combustion parameters are listed in Table 7.
| Engine specification | Operating condition | Fuel used | % of additives used | CGP | PP | HRRmax | ROPR | ID | CD | SOC | FBR | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a 4s = four stroke, NA = natural aspirated, WC = water cooled, AC = air cooled, DI = direct injection, IDI = indirect injection, CI = compression ignition, CR = compression ratio, RS = rated speed, RP = rated power, IP = injection pressure, FIP = fuel injection pressure, IT = injection timing, INS = injection system, HS = high speed, AFR = air fuel ratio, IAN = isoamyl nitrate, DTBP = di-tert-butyl peroxide, FBC = fuel-borne catalyst, DME = dimethyl ether, DEE = diethyl ether, DMC = dimethyl carbonate, DMM = dimethoxymethane, DMF = dimethylfuran, CON = cerium oxide nanoparticles, CNT = carbon nanotubes. | ||||||||||||
4s,1-Cylinder, WC, NA, DI, CR: 17.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 1500 rpm, RP: 5.2 kW, IP: 220 bar, IT: 23°, INS: pump in line nozzle injection system 1, RS: 1500 rpm, RP: 5.2 kW, IP: 220 bar, IT: 23°, INS: pump in line nozzle injection system |
Load: 25%, 50%, 75% and full load corresponding to BMEP. Speed: 1500 rpm, IP: 228 bar, IT: 23–25.5° CA BTDC | Waste cooking palm oil biodiesel | FeCl3 as a Fuel Borne Catalyst (FBC) 5–50 μmol | CGP ↑ with FBC and ↓ without FBC | PP ↑ with ↑ BMEP, in presence of FBC | HRRmax ↑ with adding FBC | — | ID ↓ with ↑ IP but as IT ↑ also ID↑ | ↑ BMEP ↓ CD, with FBC | Earlier SOC | — | 98 |
In line, 4-cylinder, IDI, NA, TC, CR: 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 4200 rpm, RP: 65 kW, IP: 157 bar, BTDC, INS: distributor type injection pump 1, RS: 4200 rpm, RP: 65 kW, IP: 157 bar, BTDC, INS: distributor type injection pump |
Torque: 80 N m constant. Speed: 1000–3000 rpm. IT: 29° CA BTDC | Jatropha biodiesel–diesel blend | 5–10% n-butanol and diethyl ether (DEE) | ↑ % of n-butanol and diethyl ether (DEE) in the blend ↓ CGP | ↑ % n-butanol ↓ PP | HRR at PC ↓ for both additives, but ↑ at DC | — | ↑ % of n-butanol and diethyl ether (DEE) ↑ ID | — | ↑ % of n-butanol and diethyl ether (DEE) ↑ SOC | — | 165 |
4s,1-Cylinder, WC, NA, DI, CR: 17.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 1500 rpm, RP: 5.2 kW, IP: 220 bar, IT: 23°, INS: pump in line nozzle injection system 1, RS: 1500 rpm, RP: 5.2 kW, IP: 220 bar, IT: 23°, INS: pump in line nozzle injection system |
Load: full load. Speed: 1200–1600 rpm | Jatropha and palm biodiesel–diesel blend | 5% by vol. of n-butanol, diethyl ether and ethanol | — | PP ↓ for both additives | HRR peak at PC ↓, but ↑ at DC | — | ID ↑ with ↑ % of additives | — | SOC delayed | — | 191 |
In line, 4-cylinder, WC, DI, TC, CR: 18.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 3600 rpm, RP: 75 kW, INS: common rail injection system 1, RS: 3600 rpm, RP: 75 kW, INS: common rail injection system |
Load: 10%, 50% and 100% of BMEP. Speed: 2400 rpm. IT: 2°, 3° and 5° CA for each load | Waste cooking oil | Ethanol (5%, 10% and 20%by vol.) | Without advanced IT, peak cylinder pressure ↑ with ↑ % ethanol at ↓ load, while with advanced IT, ↑ % ethanol ↑ the peak cylinder pressure | — | Without advanced IT, HRR ↑ as load ↑ and HRR ↓ as load ↓. Whereas, with advanced IT, HRR ↑ at all load | — | With advanced IT, ↑ % ethanol ↑ ID at low load, but ↓ ID at high load. With advanced IT and low load, ID ↑ | — | SOC Delayed as load ↓ without advanced IT | — | 181 |
In line, 4-cylinder, DI, NA, CR: 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 1800 rpm, RP: 110 kW, IT: 8° CA BTDC, INS: common rail injection system 1, RS: 1800 rpm, RP: 110 kW, IT: 8° CA BTDC, INS: common rail injection system |
Load: BMEP-0.09, 0.35 and 0.70 MPa. Torque: 30, 60, 120, 200 and 240 N m | Ultra Low Sulfur Diesel (ULSD) and biodiesel (waste cooking oil) | Ethanol (5%, 10%, and 20% by vol.) | ↓ Load: ↓ the peak in-cylinder pressures, medium load: ↑ the peak in-cylinder pressure, high load: no variation | — | ↓ Load HRR↑. At medium load HRR ↑. At high load HRRpeak ↑ than that of biodiesel but ↓ than that of ULSD | — | ID ↑ with ↑ % ethanol | CD ↓ as % ethanol ↑ | ↑ Ethanol delayed the SOC | ↑ Ethanol in DBE blends has little influence on the diffusion fuel mass | 159 |
4s,6-Cylinder, DI, NA, WC, TC, CR: 16.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 2200 rpm, INS: common rail fuel injection system 1, RS: 2200 rpm, INS: common rail fuel injection system |
Load: BMEP-0.7 MPa. IP: 160 MPa. Speed: 1330 rpm | Diesel | 2,5-Di-methylfuran, n-butanol and gasoline (30% by vol.) | D30 showed ↑ cylinder pressure | — | D30 showed ↑ HRR | D30 showed ↑ ROPRmax | ID for D30 ↑ than B30, G30 | CD for D30 ↓ than B30, G30, diesel | — | Faster mass burning rate | 158 |
4-Cylinder, DI, NA, CR: 17.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 2800 rpm, RPmax: 59 kW 1, RS: 2800 rpm, RPmax: 59 kW |
Load: BMEP-0.46 MPa, 0.58 MPa. Speed: 1800 rpm | Rapeseed oil methyl ester–diesel blends | Ethanol (10%, 20%, and 30% by volume) | — | PP ↑ with % of ethanol fraction ↑ | HRRmax ↑ with % of ethanol fraction ↑ | — | ID ↑ with % of ethanol fraction ↑ | Ethanol shortens combustion duration | — | — | 44 |
3-Cylinder, WC, NA, DI, CR: 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 1500 rpm, RP: 27.9 kW, IP: 500 bar, IT: 13° CA BTDC 1, RS: 1500 rpm, RP: 27.9 kW, IP: 500 bar, IT: 13° CA BTDC |
Load: BMEP- 0.1–0.6 MPa. Speed: 1200, 1600 rpm. IT: 13°, 18°, 21° CA BTDC | Jatropha biodiesel–diesel blend | Ethanol (20%, 30%, 40% by vol.) | Peak cylinder pressure ↓ | — | HRRmax ↑ with % of ethanol fraction ↑ | — | ID ↑ with % of ethanol fraction ↑ | — | SOC is delayed | — | 160 |
4s,1-Cylinder, WC, NA, HSDI, CR: 19.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 1000–4500 rpm. Injection advance (at pump spill): 0–40° CA 1, RS: 1000–4500 rpm. Injection advance (at pump spill): 0–40° CA |
Load: BMEP-1.40, 2.57 and 5.37 bar. Speed: 2000 rpm. IT: 29° CA BTDC | Neat cotton seed oil and its neat bio-diesel | 20% by vol. of n-butanol or diethyl ether (DEE) | CP↓ for both n-butanol and DEE at high load | — | HRR ↓ for both n-butanol and DEE | — | ID ↑ for both n-butanol and diethyl ether (DEE) | — | SOC occur later for both the n-butanol and DEE blend | — | 190 |
4s,1-Cylinder, DI, NA, CI, WC, CR: 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–20 1–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 1500 rpm, RP: 3.7 kW, FIP: 20 MPa, IT: 23° CA BTDC 1, RS: 1500 rpm, RP: 3.7 kW, FIP: 20 MPa, IT: 23° CA BTDC |
Load: BMEP-0–0.55 MPa which is from 0% to 100% load conditions. CR: 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Speed: 1500 rpm 1. Speed: 1500 rpm |
Diesterol (diesel–biodiesel–ethanol) blends. Castor oil biodiesel was used | Cerium Oxide Nanoparticles (CON) and Carbon Nanotubes (CNT) | CGP ↑ for both (CON) and (CNT) | PP ↑ for both (CON) and (CNT) | The HRR ↑ for both (CON) and (CNT) | — | ID ↓ for both (CON) and (CNT) | — | SOC occur earlier | — | 112 |
4s,1-Cylinder, DI, NA, CI, WC, CR: 16.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 2000 rpm, RP: 11.03 kW, FIP: 180 bar, IT: 22° CA BTDC 1, RS: 2000 rpm, RP: 11.03 kW, FIP: 180 bar, IT: 22° CA BTDC |
Load: BMEP-0.177 MPa, 0.531 MPa. Speed: 1500, 1800 rpm | Soybean biodiesel–diesel blends | Methanol was added by volume percent of 5% and 10% | ↑ Load ↑ % of methanol ↑ peak cylinder pressure | — | ↓ Load ↑ HRR, but at medium load HRR is similar to biodiesel, and as load ↑ HRR ↑ for both blends | ↓ Load showed similar ROPR to biodiesel, but at medium load ROPR ↓, and as load ↑ ROPR ↑ for both blends | ID ↑ at low load, but with ↑ load ID ↓ | — | SOC for both blends is later ↓ load, but is almost identical at high engine loads | — | 164 |
4s,1-Cylinder, DI, NA, CI, AC, CR: 17.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 1500 rpm, RP: 4.4 kW, FIP: 180 bar, IT: 23.4° CA BTDC 1, RS: 1500 rpm, RP: 4.4 kW, FIP: 180 bar, IT: 23.4° CA BTDC |
Load: 25%, 50%, 75% and full load corresponding to BMEP. Speed: 1500 rpm | Diesel–soybean biodiesel ethanol blends | Alumina as nano additive | Addition of alumina as nano additive ↑ CGP | PP ↑ for D80SBD15E4S1+ alumina fuel blends at all the loads respectively | Heat release rate ↑ with addition of alumina as nano additive | — | ID ↑ at no load, but as load ↑, the ignition delay ↓ | — | — | 162 | |
4s,6-Cylinder, WC, NA, DI, TC, CR: 18.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 2200 rpm, RP: 184 kW 1, RS: 2200 rpm, RP: 184 kW |
Load: BMEP-1.52 MPa. Speed: 1400 rpm | Used fried oil (UFO) | Dimethyl ether (DME) (50%, 70%, 100%) | ↑ DME ↓ peak in-cylinder pressure | — | ↑ DME ↓ HRR | ↑ DME ↓ ROPR | ↑ DME ↓ ID | — | SOC occur earlier | — | 193 |
4s,4-Cylinder, in-line, WC, NA, DI, TC, CR: 17.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: rpm, RP: 70 kW (max), IT: 12° CA BTDC 1, RS: rpm, RP: 70 kW (max), IT: 12° CA BTDC |
IT: 12° CA BTDC | Karanja oil (B100) | 10% methanol | Peak cylinder pressure ↓ with addition of methanol | — | Peak HRR ↓ with addition of methanol | Addition of methanol ↑ the ROPRmax | Addition of methanol ↑ ID | Addition of methanol ↓ CD | SOC delayed | — | 169 |
| 4s,4-Cylinder, in-line, WC, NA, IDI, RS: 1500–3500 rpm | Load: BMEP- (0.05 MPa). Speed: 2500 rpm | Biodiesel (B20) | Methanol (5% by volume) | ↑ Methanol slightly ↑ the in-cylinder pressure | — | HRR ↑ with methanol addition | Methanol addition ↑ ROPRmax | ID ↓ with methanol addition | Methanol addition ↓ CD | Delayed SOC | — | 167 |
1-Cylinder, WC, NA, DI, CR: 17.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 1500 rpm, RP: 5.2 kW, IT: 21° CA BTDC 1, RS: 1500 rpm, RP: 5.2 kW, IT: 21° CA BTDC |
Load: five engine load. Speed: 1500 rpm. Torquemax: 170 N m. | Cotton seed oil biodiesel | Ethanol (5%, 10%, and 15% by volume) | ↑ Ethanol ↑ the in cylinder pressure | — | ↑ Ethanol ↑ HRR | — | ID ↑ with ↑ ethanol | ↑ Ethanol ↓ CD | SOC was retarded | — | 166 |
4s,4-Cylinder, WC, NA, DI, HS, CR: 18.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 3400 rpm, RP: 58.5 kW, INS: pump line nozzle system 1, RS: 3400 rpm, RP: 58.5 kW, INS: pump line nozzle system |
Speed: 3400 rpm | Diesel | Ethanol, dimethyl carbonate (DMC) and di-methoxy methane (DMM) | — | — | HRRmax ↓ with ↑ % of ethanol and DMC at low load. HRRmax ↑ with ↑ % of DMC and ethanol at high load | — | ID ↑ with ↑ % of DMC and ethanol | CD ↓ with ↑ % of DMC and ethanol | — | — | 128 |
4s,1-Cylinder, WC, NA, DI, CR: 17.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 1500 rpm, RP: 5.2 kW, IP: 220 bar, IT: 23° CA BTDC, INS: mechanical pump-nozzle injection 1, RS: 1500 rpm, RP: 5.2 kW, IP: 220 bar, IT: 23° CA BTDC, INS: mechanical pump-nozzle injection |
Load: 20% to 100% in increments of 20% corresponding to BMEP. Speed: 1500 rpm | Pine oil–diesel blend | IAN – 1.5%, DTBP – 1.5% | — | — | HRRpeak ↓ for adding both IAN and DTBP | — | ID ↓ for adding both IAN and DTBP | — | Both IAN and DTBP have prompted early SOC | FBR ↓ in PC for both IAN and DTBP | 138 |
4s,1-Cylinder, WC, NA, DI, HS, CR: 15.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 1500 rpm, RP: 5.9 kW, IT: 26° CA BTDC 1, RS: 1500 rpm, RP: 5.9 kW, IT: 26° CA BTDC |
Speed: 1200 rpm. IT: 12.6° CA BTDC. Torque: 0, 4, 8, 12, 16, 20 and 24 N m | Jatropha–diesel blends | Ethanol (5% by vol.) | Higher % of JME with 5% ethanol, ↓ the peak pressure | — | HRRpeak ↑ with adding ethanol | — | Higher % of JME with 5% ethanol, ↑ ID | ↑ % JME with 5% ethanol, ↑ CD | ↓ % JME with 5% ethanol, advanced the SOC | — | 157 |
1-Cylinder, WC, NA, DI, CR: 15.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 2500 rpm, RPmax: 8.6 kW, INS: mechanical injection system 1, RS: 2500 rpm, RPmax: 8.6 kW, INS: mechanical injection system |
Load: IMEP-3 bar, EGR: 0%, 10 and 20%. Speed: 1500 rpm | Rapeseed oil methyl ester (15%) and ultra low sulfur diesel (ULSD) | Ethanol (10%) and butanol (16%) | — | — | With addition of both ethanol and butanol ↑ HRRpeak | — | — | ID ↑ with addition of both ethanol and butanol | SOC delayed with addition of both ethanol and butanol | — | 192 |
4s,1-Cylinder, NA, DI, CR: 16.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 1500 rpm, RP: 3.5 kW, INS: three hole nozzle injection system 1, RS: 1500 rpm, RP: 3.5 kW, INS: three hole nozzle injection system |
Speed: 1500 rpm | Neem oil methyl ester | Diethyl ether (5%, 10% and 15% by vol.) | ↑ % DEE ↓ cylinder pressure | — | ↑ % DEE ↓ HRRmax | — | ↑ % DEE ↓ ID | — | — | — | 20 |
4s, In line, 4-cylinder, DI, NA, WC, CR: 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 2200 rpm, RP: 60 kW, IT: 25° CA BTDC 1, RS: 2200 rpm, RP: 60 kW, IT: 25° CA BTDC |
Speed: 1400, 1800, 2200 rpm. AFR: air–fuel ratios of λ = 5.5, 3.0 and 1.5 | Ethanol (5%, 10%, and 15% by volume) and diesel | Rapeseed oil methyl ester (5%) | In cylinder pressure ↑ with RME and ethanol | — | HRRmax ↑ with RME and ethanol | — | ID ↑ with RME and ethanol | — | SOC occur earlier | — | 161 |
4s,1-Cylinder, NA, DI, CI, CR: 17.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, RS: 1500 rpm, RP: 5.2 kW, IP: 220 bar, IT: 23° CA BTDC, INS: mechanical pump-nozzle injection 1, RS: 1500 rpm, RP: 5.2 kW, IP: 220 bar, IT: 23° CA BTDC, INS: mechanical pump-nozzle injection |
Load: varied in steps of 20% from 20% to 100%, corresponding to torque. Speed: 1500 rpm | Kapok methyl ester with diesel. B25(25% KME and 75% diesel) | 1,4-Dioxane (0.5%, 1.0%) | — | — | ↑ % 1,4-dioxane ↓ HRR peak | — | ↑ % 1,4-dioxane ID ↓ | — | SOC occur earlier | FBR ↓ in PC and ↑ in DC | 168 |
7. Summary
• Metal-based additives such as FeCl3, CON, CNT, alumina nanoparticles showed an increased in HRR, CGP and PP with earlier SOC and decreased ID. Presence of oxygen content, better fuel mixing, increased oxidation of hydrocarbons and improved combustion rate in both combustion zone are mainly attributed to this. Introducing metal-based additives provide more oxygen to prompt the combustion.• Increased proportion of n-butanol, DEE in biodiesel decreases the CGP and PP whereas HRR decreased at premixed combustion (PC) and increased in diffusion combustion (DC) increased the ID with delayed SOC. Higher latent heat of evaporation, lower dynamic injection timing leads to delayed SOC, although DEE increases cetane number. More ideal fuel atomization and vaporization were found. But there was an exception in case of DEE with Neem biodiesel as decreased ID with increasing proportion of DEE in the blends. This is because of less air–fuel mixture formation during ID period, higher cetane number and lower cylinder pressure.
• Increase in ethanol percentage in the blends increases the cylinder pressure but decreases with high ethanol content in the blends due to cooling effect of higher blends. The PP, HRRmax and ID also increases with increased ethanol in the blends. Lower calorific value, lower cetane number, higher latent heat of vaporization, faster rate of evaporation of ethanol account for this behavior. Increase in residual gas temperature and cylinder wall temperature with increasing engine load, leads to elevated charge temperature before injection and shortening the ignition delays. The higher latent heat of vaporization of ethanol leads to lower in-cylinder temperature and consequently an increase in ignition delay was found. Higher oxygen content of the blends with ethanol indicates improved diffusion combustion with high heat release rate that leads to lower the combustion duration. Addition of high ethanol content lower the viscosity and gives better air–fuel mixture that helps to burn a higher amount of fuel in the premixed combustion stage.
• Addition of methanol proportion in the blends increased the cylinder pressure, HRR, ROPR with decreased ID, CD and SOC delayed. While 10% of methanol in neat karanja oil (100%) reduced the cylinder pressure, HRR with increased ID. Lower cetane number and higher viscosity is responsible for this.
• DME and 1,4-dioxane in biodiesel decreased the cylinder pressure, HRR, ROPR, ID with earlier SOC. DME showed a slight modulus of elasticity with high compressibility. Whereas 1,4-dioxane showed decreased FBR in PC and increased FBR in DC, increased oxygen content, improved fuel properties prompting the diffusion combustion and increased the heat release.
• Ignition promoter IAN and DTBP in biodiesel decreased HRR peak, ID with earlier SOC and reduced FBR in PC along with an efficient combustion. Both IAN and DTBP in diesel–biodiesel blends improved the density of biodiesel than diesel so accelerating the diffusion combustion and upgrading the fuel characteristics of biodiesel, bringing about an efficient combustion. The combustion phasing using ignition promoter are further to be investigated.
• 2,5-DMF, ethanol, DMC and DMM additives were also used as additives with diesel fuel and lead to an increase in the cylinder pressure, HRR, ROPR, ID, FBR with decreased CD. Increase in ignition delay leads to more homogeneous air–fuel mixtures and faster burning at premixed combustion stage.
8. Conclusion
The principle objectives of this review was to determine the optimum conditions of combustion from previous work upon incorporation of various proportion of additives with diesel, biodiesel and their blends and correlates them to reach an optimum operating level while keeping other conditions reasonable. Addition of additives in diesel, biodiesel and their blends has a great effect on fuel properties such as viscosity, flash point, fire point, pour point, calorific value etc. which in-turns influences the combustion parameters. Most of the oxygenated additives showed improved combustion phases, decrease in in-cylinder temperature due to high latent heat of evaporation. Multifunctional fuel additives in fuel blends decreases the ignition delay, improved premixed combustion duration and combustion stability. Ignition promoter additives improved the ignition attributes and the conditions should be further be investigated. Various types of antioxidants and cold flow improver additives will also need to be studied to ensure better combustion attributes. Combustion parameters such as ignition delay, heat release rate, rate of fuel burn, combustion phases will critically need to be analyzed with several potential additives such as five-carbon structure oxygenated additives, metal-based additives, cetane improver additives etc. Fuel properties of various proportions of additives used in diesel and biodiesel to produce blends must be investigated intensively to reduce the existing problems after incorporating additives.Nomenclature
| ATDC | After Top Dead Centre |
| BTDC | Before Top Dead Centre |
| BSFC | Break Specific Fuel Consumption |
| BTE | Break Thermal Efficiency |
| BSEC | Break Specific Energy Consumption |
| BMEP | Break Mean Effective Pressure |
| CA | Crank Angle |
| CAD | Crank Angle Degree |
| CD | Combustion Duration |
| CGP | Cylinder Gas Pressure |
| CN | Cetane Number |
| CO | Carbon Monoxide |
| DC | Diffusion Combustion |
| FBR | Fuel Burning Rate |
| HC | Hydro Carbon |
| HRR | Heat Release Rate |
| HRRmax | Maximum Heat Release Rate |
| HRRpeak | Peak Heat Release Rate |
| ID | Ignition Delay |
| NOx | Oxides of Nitrogen |
| PM | Particulate Matter |
| PC | Premixed Combustion |
| PP | Peak Pressure |
| ROPR | Rate of Pressure Rise |
| SOC | Start of Combustion |
| SOI | Start of Injection |
| TDC | Top Dead Center |
| THC | Total Hydrocarbon |
| WCO | Waste Cooking Oil |
Acknowledgements
The authors are grateful to University of Malaya for financial support through High Impact Research grant titled: “Clean Diesel Technology for Military and Civilian Transport Vehicles” having grant number UM.C/HIR/MOHE/ENG/07.References
- M. Mofijur, H. H. Masjuki, M. A. Kalam, A. E. Atabani, M. Shahabuddin, S. M. Palash and M. A. Hazrat, Renewable Sustainable Energy Rev., 2013, 28, 441–455 CrossRef CAS PubMed.
- A. Sanjid, H. H. Masjuki, M. A. Kalam, S. M. A. Rahman, M. J. Abedin and I. M. R. Fattah, RSC Adv., 2015, 5, 13246–13255 RSC.
- J. Pickett, D. Anderson, D. Bowles, T. Bridgwater, P. Jarvis, N. Mortimer, M. Poliakoff and J. Woods, Sustainable biofuels: prospects and challenges, Policy document 01/08, The Royal Society, ISBN 978 0 85403 662 2, 2008 Search PubMed.
- R. Luque, L. Herrero-Davila, J. M. Campelo, J. H. Clark, J. M. Hidalgo, D. Luna, J. M. Marinas and A. A. Romero, Energy Environ. Sci., 2008, 1, 542–564 CAS.
- Y. H. Teoh, H. H. Masjuki, M. A. Kalam, M. A. Amalina and H. G. How, RSC Adv., 2014, 4, 50739–50751 RSC.
- B. M. Masum, H. H. Masjuki, M. A. Kalam, S. M. Palash, M. A. Wakil and S. Imtenan, Energy Convers. Manage., 2014, 88, 382–390 CrossRef CAS PubMed.
- B. Masum, M. Kalam, H. Masjuki, S. A. Rahman and E. Daggig, RSC Adv., 2014, 4, 51220–51227 RSC.
- A. M. Ashraful, H. H. Masjuki, M. A. Kalam, H. K. Rashedul, H. Sajjad and M. J. Abedin, Energy Convers. Manage., 2014, 87, 48–57 CrossRef CAS PubMed.
- O. Özener, L. Yüksek, A. T. Ergenç and M. Özkan, Fuel, 2014, 115, 875–883 CrossRef PubMed.
- X. Wang, Y. Ge, L. Yu and X. Feng, Fuel, 2013, 107, 852–858 CrossRef CAS PubMed.
- G. Sakthivel, G. Nagarajan, M. Ilangkumaran and A. B. Gaikwad, Fuel, 2014, 132, 116–124 CrossRef CAS PubMed.
- H. G. How, H. H. Masjuki, M. A. Kalam and Y. H. Teoh, Energy, 2014, 69, 749–759 CrossRef CAS PubMed.
- I. M. Rizwanul Fattah, M. A. Kalam, H. H. Masjuki and M. A. Wakil, RSC Adv., 2014, 4, 17787–17796 RSC.
- M. I. Arbab, H. H. Masjuki, M. Varman, M. A. Kalam, S. Imtenan and H. Sajjad, Renewable Sustainable Energy Rev., 2013, 22, 133–147 CrossRef CAS PubMed.
- M. Mofijur, H. H. Masjuki, M. A. Kalam, A. E. Atabani, M. I. Arbab, S. F. Cheng and S. W. Gouk, Energy Convers. Manage., 2014, 82, 169–176 CrossRef CAS PubMed.
- M. Hajjari, M. Ardjmand and M. Tabatabaei, RSC Adv., 2014, 4, 14352–14356 RSC.
- H. K. Rashedul, H. H. Masjuki, M. A. Kalam, A. M. Ashraful, M. M. Rashed, I. Sanchita and T. Shaon, RSC Adv., 2014, 4, 64791–64797 RSC.
- S. Imtenan, H. H. Masjuki, M. Varman and I. M. Rizwanul Fattah, RSC Adv., 2015, 5, 17160–17170 RSC.
- M. I. Arbab, H. H. Masjuki, M. Varman, M. A. Kalam, H. Sajjad and S. Imtenan, RSC Adv., 2014, 4, 37122–37129 RSC.
- S. Sivalakshmi and T. Balusamy, Fuel, 2013, 106, 106–110 CrossRef CAS PubMed.
- M. Mofijur, A. E. Atabani, H. H. Masjuki, M. A. Kalam and B. M. Masum, Renewable Sustainable Energy Rev., 2013, 23, 391–404 CrossRef CAS PubMed.
- M. M. Rahman, M. H. Hassan, M. A. Kalam, A. E. Atabani, L. A. Memon and S. M. A. Rahman, J. Clean. Prod., 2014, 65, 304–310 CrossRef CAS PubMed.
- S. H. Shuit, K. T. Tan, K. T. Lee and A. H. Kamaruddin, Energy, 2009, 34, 1225–1235 CrossRef CAS PubMed.
- N. M. Ribeiro, A. C. Pinto, C. M. Quintella, G. O. da Rocha, L. S. G. Teixeira, L. L. N. Guarieiro, M. do Carmo Rangel, M. C. C. Veloso, M. J. C. Rezende, R. Serpa da Cruz, A. M. de Oliveira, E. A. Torres and J. B. de Andrade, Energy Fuels, 2007, 21, 2433–2445 CrossRef CAS.
- M. Shahabuddin, A. M. Liaquat, H. H. Masjuki, M. A. Kalam and M. Mofijur, Renewable Sustainable Energy Rev., 2013, 21, 623–632 CrossRef CAS PubMed.
- M. Gumus, Fuel, 2010, 89, 2802–2814 CrossRef CAS PubMed.
- S. Lahane and K. A. Subramanian, Fuel, 2015, 139, 537–545 CrossRef CAS PubMed.
- J. Sun, J. A. Caton and T. J. Jacobs, Prog. Energy Combust. Sci., 2010, 36, 677–695 CrossRef CAS PubMed.
- L.-Y. Chen, Y.-H. Chen, Y.-S. Hung, T.-H. Chiang and C.-H. Tsai, J. Taiwan Inst. Chem. Eng., 2013, 44, 214–220 CrossRef CAS PubMed.
- N. Usta, Energy Convers. Manage., 2005, 46, 2373–2386 CrossRef CAS PubMed.
- A. Dhar, R. Kevin and A. K. Agarwal, Fuel Process. Technol., 2012, 97, 118–129 CrossRef CAS PubMed.
- T. T. Al-Shemmeri and S. Oberweis, Appl. Therm. Eng., 2011, 31, 1682–1688 CrossRef CAS PubMed.
- P. C. Smith, Y. Ngothai, Q. Dzuy Nguyen and B. K. O’Neill, Renewable Energy, 2010, 35, 1145–1151 CrossRef CAS PubMed.
- T. F. Yusaf, B. F. Yousif and M. M. Elawad, Energy, 2011, 36, 4871–4878 CrossRef CAS PubMed.
- E. Kinoshita, K. Hamasaki, C. Jaqin and K. Takasaki, Combustion characteristics for diesel engines with emulsified biodiesel without adding emulsifier, SAE Tech. Pap. Ser., 2004 Search PubMed.
- M. Iranmanesh, J. Subrahmanyam and M. Babu, Potential of diethyl ether as a blended supplementary oxygenated fuel with biodiesel to improve combustion and emission characteristics of diesel engines, SAE Tech. Pap. Ser., 2008 Search PubMed.
- H. Li, A. Lea-Langton, P. Biller, G. E. Andrews, S. Hadavi, A. Charlton and P. Richards, Effect of Multifunctional Fuel Additive Package on Fuel Injector Deposit, Combustion and Emissions using Pure Rape Seed Oil for a DI Diesel, SAE Tech. Pap. Ser., 2009 Search PubMed.
- W. W. Pulkrabek, Engineering fundamentals of the internal combustion engine, Prentice Hall Upper Saddle River, NJ, 1997 Search PubMed.
- G. A. Ban-Weiss, J. Y. Chen, B. A. Buchholz and R. W. Dibble, Fuel Process. Technol., 2007, 88, 659–667 CrossRef CAS PubMed.
- V. Ganesan, Internal combustion engines, McGraw Hill Education (India) Pvt Ltd, 2012 Search PubMed.
- R. Stone, Introduction to internal combustion engines, Basingstoke, UK, 1999 Search PubMed.
- X. Sun, W. G. Wang, R. M. Bata and X. Gao, J. Eng. Gas Turbines Power, 1994, 116, 758–764 CrossRef.
- K. Kohse-Höinghaus, P. Oßwald, T. A. Cool, T. Kasper, N. Hansen, F. Qi, C. K. Westbrook and P. R. Westmoreland, Angew. Chem., Int. Ed., 2010, 49, 3572–3597 CrossRef PubMed.
- H. Chen, S. Shi-Jin and W. Jian-Xin, Proc. Combust. Inst., 2007, 31, 2981–2989 CrossRef PubMed.
- A. K. Agarwal, Prog. Energy Combust. Sci., 2007, 33, 233–271 CrossRef CAS PubMed.
- T. Fang and C.-f. F. Lee, Proc. Combust. Inst., 2009, 32, 2785–2792 CrossRef CAS PubMed.
- M. Lapuerta, O. Armas and J. Rodríguez-Fernández, Prog. Energy Combust. Sci., 2008, 34, 198–223 CrossRef CAS PubMed.
- M. M. Rashed, M. A. Kalam, H. H. Masjuki, H. K. Rashedul, A. M. Ashraful, I. Shancita and A. M. Ruhul, RSC Adv., 2015, 5, 36240–36261 RSC.
- A. Demirbas, Prog. Energy Combust. Sci., 2007, 33, 1–18 CrossRef CAS PubMed.
- C. K. Westbrook, W. J. Pitz, P. R. Westmoreland, F. L. Dryer, M. Chaos, P. Osswald, K. Kohse-Höinghaus, T. A. Cool, J. Wang, B. Yang, N. Hansen and T. Kasper, Proc. Combust. Inst., 2009, 32, 221–228 CrossRef CAS PubMed.
- P. Dagaut, S. Gaïl and M. Sahasrabudhe, Proc. Combust. Inst., 2007, 31, 2955–2961 CrossRef PubMed.
- O. Herbinet, W. J. Pitz and C. K. Westbrook, Combust. Flame, 2008, 154, 507–528 CrossRef CAS PubMed.
- C. J. Hayes and D. R. Burgess Jr, Proc. Combust. Inst., 2009, 32, 263–270 CrossRef CAS PubMed.
- K. Seshadri, T. Lu, O. Herbinet, S. Humer, U. Niemann, W. J. Pitz, R. Seiser and C. K. Law, Proc. Combust. Inst., 2009, 32, 1067–1074 CrossRef CAS PubMed.
- P. Osswald, U. Struckmeier, T. Kasper, K. Kohse-Höinghaus, J. Wang, T. A. Cool, N. Hansen and P. R. Westmoreland, J. Phys. Chem. A, 2007, 111, 4093–4101 CrossRef CAS PubMed.
- W. K. Metcalfe, C. Togbé, P. Dagaut, H. J. Curran and J. M. Simmie, Combust. Flame, 2009, 156, 250–260 CrossRef CAS PubMed.
- O. Herbinet, W. J. Pitz and C. K. Westbrook, Combust. Flame, 2010, 157, 893–908 CrossRef CAS PubMed.
- A. K. Agarwal, A. Dhar, J. G. Gupta, W. I. Kim, K. Choi, C. S. Lee and S. Park, Energy Convers. Manage., 2015, 91, 302–314 CrossRef CAS PubMed.
- H. K. Suh, H. G. Roh and C. S. Lee, J. Eng. Gas Turbines Power, 2008, 130, 032807 CrossRef.
- B. Aliyu, D. Shitanda, S. Walker, B. Agnew, S. Masheiti and R. Atan, Appl. Therm. Eng., 2011, 31, 36–41 CrossRef CAS PubMed.
- S. Lee, S. Oh, Y. Choi and K. Kang, Appl. Therm. Eng., 2011, 31, 1929–1935 CrossRef CAS PubMed.
- E. Buyukkaya, Fuel, 2010, 89, 3099–3105 CrossRef CAS PubMed.
- D. H. Qi, H. Chen, L. M. Geng and Y. Z. Bian, Energy Convers. Manage., 2010, 51, 2985–2992 CrossRef CAS PubMed.
- S. Pinzi, P. Rounce, J. M. Herreros, A. Tsolakis and M. Pilar Dorado, Fuel, 2013, 104, 170–182 CrossRef CAS PubMed.
- C. C. Enweremadu and H. L. Rutto, Renewable Sustainable Energy Rev., 2010, 14, 2863–2873 CrossRef CAS PubMed.
- M. Canakci and H. Sanli, J. Ind. Microbiol. Biotechnol., 2008, 35, 431–441 CrossRef CAS PubMed.
- L. Labecki, A. Cairns, J. Xia, A. Megaritis, H. Zhao and L. C. Ganippa, Appl. Energy, 2012, 95, 139–146 CrossRef CAS PubMed.
- A. Abu-Jrai, J. A. Yamin, A. a. H. Al-Muhtaseb and M. A. Hararah, Chem. Eng. J., 2011, 172, 129–136 CrossRef CAS PubMed.
- P. Benjumea, J. R. Agudelo and A. F. Agudelo, Energy Fuels, 2011, 25, 77–85 CrossRef CAS.
- M. Mofijur, H. H. Masjuki, M. A. Kalam and A. E. Atabani, Energy, 2013, 55, 879–887 CrossRef CAS PubMed.
- S. Puhan, N. Saravanan, G. Nagarajan and N. Vedaraman, Biomass Bioenergy, 2010, 34, 1079–1088 CrossRef CAS PubMed.
- H. Song, B. T. Tompkins, J. A. Bittle and T. J. Jacobs, Fuel, 2012, 96, 446–453 CrossRef CAS PubMed.
- E. Öztürk, Fuel Process. Technol., 2015, 129, 183–191 CrossRef PubMed.
- A. L. Boehman, D. Morris, J. Szybist and E. Esen, Energy Fuels, 2004, 18, 1877–1882 CrossRef CAS.
- S. M. Palash, H. H. Masjuki, M. A. Kalam, B. M. Masum, A. Sanjid and M. J. Abedin, Energy Convers. Manage., 2013, 76, 400–420 CrossRef CAS PubMed.
- E. Sher, Handbook of air pollution from internal combustion engines: pollutant formation and control, Academic Press, 1998 Search PubMed.
- M. Gürü, A. Koca, Ö. Can, C. Çınar and F. Şahin, Renewable Energy, 2010, 35, 637–643 CrossRef PubMed.
- C. D. Rakopoulos, K. A. Antonopoulos and D. C. Rakopoulos, Energy Convers. Manage., 2006, 47, 1550–1573 CrossRef CAS PubMed.
- C. J. Mueller, A. L. Boehman and G. C. Martin, An experimental investigation of the origin of increased NOx emissions when fueling a heavy-duty compression–ignition engine with soy biodiesel, SAE Tech. Pap. Ser., 2009 Search PubMed.
- A. N. Ozsezen and M. Canakci, Biomass Bioenergy, 2010, 34, 1870–1878 CrossRef CAS PubMed.
- M. Zhihao, Z. Xiaoyu, D. Junfa, W. Xin, X. Bin and W. Jian, Procedia Environ. Sci., 2011, 11, 1078–1083 CrossRef PubMed.
- T. Ganapathy, R. P. Gakkhar and K. Murugesan, Appl. Energy, 2011, 88, 4376–4386 CrossRef CAS PubMed.
- B. Challen and R. Baranescu, Diesel engine reference book, SAE and Butterworth Heinemann, Woburn, MA, 1999 Search PubMed.
- A. N. Ozsezen and M. Canakci, Journal of the Faculty of Engineering and Architecture of Gazi University, 2008, 23, 395–404 Search PubMed.
- J. P. Szybist, J. Song, M. Alam and A. L. Boehman, Fuel Process. Technol., 2007, 88, 679–691 CrossRef CAS PubMed.
- N. Usta, E. Öztürk, Ö. Can, E. S. Conkur, S. Nas, A. H. Çon, A. Ç. Can and M. Topcu, Energy Convers. Manage., 2005, 46, 741–755 CrossRef CAS PubMed.
- N. Usta, Biomass Bioenergy, 2005, 28, 77–86 CrossRef CAS PubMed.
- P.-q. Tan, Z.-y. Hu, D.-m. Lou and Z.-j. Li, Energy, 2012, 39, 356–362 CrossRef CAS PubMed.
- C. Sayin, M. Gumus and M. Canakci, Energy Fuels, 2010, 24, 2675–2682 CrossRef CAS.
- Ö. Can, Energy Convers. Manage., 2014, 87, 676–686 CrossRef PubMed.
- A. N. Ozsezen, M. Canakci, A. Turkcan and C. Sayin, Fuel, 2009, 88, 629–636 CrossRef CAS PubMed.
- A. S. Silitonga, H. H. Masjuki, T. M. I. Mahlia, H. C. Ong and W. T. Chong, Energy Convers. Manage., 2013, 76, 828–836 CrossRef CAS PubMed.
- S. H. Park, J. Cha and C. S. Lee, Appl. Energy, 2012, 99, 334–343 CrossRef CAS PubMed.
- B. T. Tompkins, H. Song, J. A. Bittle and T. J. Jacobs, Appl. Energy, 2012, 98, 209–218 CrossRef CAS PubMed.
- M. Gumus, C. Sayin and M. Canakci, Fuel, 2012, 95, 486–494 CrossRef CAS PubMed.
- H. K. Rashedul, H. H. Masjuki, M. A. Kalam, A. M. Ashraful, S. M. Ashrafur Rahman and S. A. Shahir, Energy Convers. Manage., 2014, 88, 348–364 CrossRef CAS PubMed.
- R. D. Misra and M. S. Murthy, Renewable Sustainable Energy Rev., 2011, 15, 2413–2422 CrossRef CAS PubMed.
- G. R. Kannan, R. Karvembu and R. Anand, Appl. Energy, 2011, 88, 3694–3703 CrossRef CAS PubMed.
- M. A. Kalam and H. H. Masjuki, Biomass Bioenergy, 2008, 32, 1116–1122 CrossRef CAS PubMed.
- A. Keskin, M. Gürü and D. Altıparmak, Fuel, 2007, 86, 1139–1143 CrossRef CAS PubMed.
- K.-S. Chen, Y.-C. Lin, L.-T. Hsieh, L.-F. Lin and C.-C. Wu, Energy, 2010, 35, 2043–2048 CrossRef CAS PubMed.
- S. A. Basha and K. Raja Gopal, Renewable Sustainable Energy Rev., 2012, 16, 711–717 CrossRef CAS PubMed.
- A. Groysman, Corrosion in Systems for Storage and Transportation of Petroleum Products and Biofuels: Identification, Monitoring and Solutions, Springer Science & Business Media, 2014 Search PubMed.
- https://4plusfueltreatment.wordpress.com/2013/01/01/pros-and-cons-of-using-fuel-additives/, 2015.
- http://www.fuel-testers.com/ethanol_problems_damage.html, 2015.
- http://www.solarpowernotes.com/renewable-energy/ethanol-fuel.html, 2015.
- N. M. Ribeiro, A. C. Pinto, C. M. Quintella, G. O. Da Rocha, L. S. Teixeira, L. L. Guarieiro, M. do Carmo Rangel, M. C. Veloso, M. J. Rezende and R. Serpa da Cruz, Energy Fuels, 2007, 21, 2433–2445 CrossRef CAS.
- T. Campenon, P. Wouters, G. Blanchard, P. Macaudiere and T. Seguelong, Improvement and simplification of DPF system using a ceria-based fuel-borne catalyst for diesel particulate filter regeneration in serial applications, SAE Tech. Pap. Ser., 2004 Search PubMed.
- H.-H. Yang, W.-J. Lee, H.-H. Mi, C.-H. Wong and C.-B. Chen, Environ. Int., 1998, 24, 389–403 CrossRef CAS.
- M. Kasper, K. Sattler, K. Siegmann, U. Matter and H. C. Siegmann, J. Aerosol Sci., 1999, 30, 217–225 CrossRef CAS.
- H. Burtscher, U. Matter and G. Skillas, J. Aerosol Sci., 1999, 30(suppl. 1), S851–S852 CrossRef.
- V. Arul Mozhi Selvan, R. B. Anand and M. Udayakumar, Fuel, 2014, 130, 160–167 CrossRef CAS PubMed.
- http://www.chemspider.com/, 2015.
- N. Rahmat, A. Z. Abdullah and A. R. Mohamed, Renewable Sustainable Energy Rev., 2010, 14, 987–1000 CrossRef CAS PubMed.
- M. Lapuerta, R. García-Contreras, J. Campos-Fernández and M. P. Dorado, Energy Fuels, 2010, 24, 4497–4502 CrossRef CAS.
- E. Coda Zabetta, M. Hupa and S. Niemi, Fuel, 2006, 85, 2666–2670 CrossRef CAS PubMed.
- E. Weber de Menezes, R. da Silva, R. Cataluña and R. J. C. Ortega, Fuel, 2006, 85, 815–822 CrossRef CAS PubMed.
- C. Y. Lin and J. C. Huang, Ocean Eng., 2003, 30, 1699–1715 CrossRef.
- T. Kitamura, T. Ito, J. Senda and H. Fujimoto, JSAE Rev., 2001, 22, 139–145 CrossRef CAS.
- A. C. Hansen, Q. Zhang and P. W. L. Lyne, Bioresour. Technol., 2005, 96, 277–285 CrossRef CAS PubMed.
- P. Satgé de Caro, Z. Mouloungui, G. Vaitilingom and J. C. Berge, Fuel, 2001, 80, 565–574 CrossRef.
- B.-Q. He, S.-J. Shuai, J.-X. Wang and H. He, Atmos. Environ., 2003, 37, 4965–4971 CrossRef CAS PubMed.
- E. E. Ecklund, R. L. Bechtold, T. J. Timbario and P. W. McCallum, State-of-the-art report on the use of alcohols in diesel engines, Report 0148–7191, SAE Tech. Pap. Ser., 1984 Search PubMed.
- Y. C. Sharma, B. Singh and S. N. Upadhyay, Fuel, 2008, 87, 2355–2373 CrossRef CAS PubMed.
- S. A. Basha, K. R. Gopal and S. Jebaraj, Renewable Sustainable Energy Rev., 2009, 13, 1628–1634 CrossRef CAS PubMed.
- E. Broukhiyan and S. Lestz, Ethanol fumigation of a light duty automotive diesel engine, SAE Tech. Pap. Ser., 1981 Search PubMed.
- T. L. Ullman, K. B. Spreen and R. L. Mason, Effects of cetane number, cetane improver, aromatics, and oxygenates on 1994 heavy-duty diesel engine emissions, SAE Tech. Pap. Ser., 1994 Search PubMed.
- X.-c. Lü, J.-g. Yang, W.-g. Zhang and Z. Huang, Energy Fuels, 2005, 19, 1879–1888 CrossRef.
- A. S. Ramadhas, S. Jayaraj, C. Muraleedharan and K. Padmakumari, Renewable Energy, 2006, 31, 2524–2533 CrossRef CAS PubMed.
- J. Bennett, in Alternative Fuels and Advanced Vehicle Technologies for Improved Environmental Performance, ed. R. Folkson, Woodhead Publishing, 2014, pp. 165–194 Search PubMed.
- G. Knothe and K. Carlson, J. Am. Oil Chem. Soc., 1998, 75, 1861–1866 CrossRef CAS.
- M. A. Hess, M. J. Haas, T. A. Foglia and W. N. Marmer, Prepr. Pap. - Am. Chem. Soc., Div. Fuel Chem., 2004, 49, 852 CAS.
- G. J. Suppes, M. Goff, M. L. Burkhart, K. Bockwinkel, M. H. Mason, J. B. Botts and J. A. Heppert, Energy Fuels, 2001, 15, 151–157 CrossRef CAS.
- S. A. Zinenko, S. A. Egorov, A. A. Makarov, E. A. Sharin, V. M. Manaenkov and A. M. Bakaleinik, Chem. Technol. Fuels Oils, 2002, 38, 303–308 CrossRef CAS.
- A. M. Danilov, Chem. Technol. Fuels Oils, 2001, 37, 444–455 CrossRef CAS.
- J. F. Schabron and M. P. Fuller, Anal. Chem., 1982, 54, 2599–2601 CrossRef CAS.
- L. Guo, Y. Y. Yan, M. Tan, H. Li and Y. Peng, Chem. Eng. Commun., 2011, 198, 1263–1274 CrossRef CAS PubMed.
- R. Vallinayagam, S. Vedharaj, W. M. Yang, C. G. Saravanan, P. S. Lee, K. J. E. Chua and S. K. Chou, Fuel, 2014, 117, 278–285 CrossRef CAS PubMed.
- X. Lang, A. K. Dalai, N. N. Bakhshi, M. J. Reaney and P. B. Hertz, Bioresour. Technol., 2001, 80, 53–62 CrossRef CAS.
- J. M. Hughes, G. W. Mushrush and D. R. Hardy, Ind. Eng. Chem. Res., 2002, 41, 1386–1388 CrossRef CAS.
- D. Karonis, G. Anastopoulos, E. Lois, S. Stournas, F. Zannikos and A. Serdari, Assessment of the lubricity of Greek road diesel and the effect of the addition of specific types of biodiesel, SAE Tech. Pap. Ser., 1999 Search PubMed.
- A. K. Bhatnagar, S. Kaul, V. K. Chhibber and A. K. Gupta, Energy Fuels, 2006, 20, 1341–1344 CrossRef CAS.
- R. J. Batt, J. A. McMillan and I. Bradbury, Lubricity additives-performance and no-harm effects in low sulfur fuels, SAE Tech. Pap. Ser., 1996 Search PubMed.
- G. Anastopoulos, E. Lois, F. Zannikos, S. Kalligeros and C. Teas, Tribol. Int., 2001, 34, 749–755 CrossRef CAS.
- D. P. Geller and J. W. Goodrum, Fuel, 2004, 83, 2351–2356 CrossRef CAS PubMed.
- G. Joshi, B. Y. Lamba, D. S. Rawat, S. Mallick and K. S. R. Murthy, Ind. Eng. Chem. Res., 2013, 52, 7586–7592 CrossRef CAS.
- G. Karavalakis, D. Hilari, L. Givalou, D. Karonis and S. Stournas, Energy, 2011, 36, 369–374 CrossRef CAS PubMed.
- S. Schober and M. Mittelbach, Eur. J. Lipid Sci. Technol., 2004, 106, 382–389 CrossRef CAS PubMed.
- R. L. McCormick, M. Ratcliff, L. Moens and R. Lawrence, Fuel Process. Technol., 2007, 88, 651–657 CrossRef CAS PubMed.
- S. Jain and M. P. Sharma, Renewable Sustainable Energy Rev., 2010, 14, 667–678 CrossRef CAS PubMed.
- I. M. Rizwanul Fattah, H. H. Masjuki, M. A. Kalam, M. Mofijur and M. J. Abedin, Energy Convers. Manage., 2014, 79, 265–272 CrossRef CAS PubMed.
- S. M. Sarathy, P. Oßwald, N. Hansen and K. Kohse-Höinghaus, Prog. Energy Combust. Sci., 2014, 44, 40–102 CrossRef PubMed.
- I. M. Rizwanul Fattah, H. H. Masjuki, M. A. Kalam, M. A. Wakil, H. K. Rashedul and M. J. Abedin, Ind. Crops Prod., 2014, 57, 132–140 CrossRef CAS PubMed.
- M. Shahabuddin, M. A. Kalam, H. H. Masjuki, M. M. K. Bhuiya and M. Mofijur, Energy, 2012, 44, 616–622 CrossRef CAS PubMed.
- M. Canakci, Bioresour. Technol., 2007, 98, 1167–1175 CrossRef CAS PubMed.
- D. C. Rakopoulos, C. D. Rakopoulos, E. G. Giakoumis, R. G. Papagiannakis and D. C. Kyritsis, Energy, 2014, 73, 354–366 CrossRef CAS PubMed.
- D. Kannan, S. Pachamuthu, M. Nurun Nabi, J. E. Hustad and T. Løvås, Energy Convers. Manage., 2012, 53, 322–331 CrossRef CAS PubMed.
- G. Chen, Y. Shen, Q. Zhang, M. Yao, Z. Zheng and H. Liu, Energy, 2013, 54, 333–342 CrossRef CAS PubMed.
- H. Tse, C. W. Leung and C. S. Cheung, Energy, 2015, 83, 343–350 CrossRef CAS PubMed.
- D. B. Hulwan and S. V. Joshi, Appl. Energy, 2011, 88, 5042–5055 CrossRef CAS PubMed.
- G. Labeckas, S. Slavinskas and M. Mažeika, Energy Convers. Manage., 2014, 79, 698–720 CrossRef CAS PubMed.
- T. Shaafi and R. Velraj, Renewable Energy, 2015, 80, 655–663 CrossRef CAS PubMed.
- D. H. Qi, H. Chen, L. M. Geng and Y. Z. Bian, Renewable Energy, 2011, 36, 1252–1258 CrossRef CAS PubMed.
- D. H. Qi, H. Chen, L. M. Geng, Y. Z. Bian and X. C. Ren, Appl. Energy, 2010, 87, 1679–1686 CrossRef CAS PubMed.
- S. Imtenan, H. H. Masjuki, M. Varman, I. M. Rizwanul Fattah, H. Sajjad and M. I. Arbab, Energy Convers. Manage., 2015, 94, 84–94 CrossRef CAS PubMed.
- A. Anbarasu, M. Saravanan and M. Loganathan, Int. J. Green Energy, 2013, 10, 90–102 CrossRef CAS PubMed.
- M. H. Mat Yasin, T. Yusaf, R. Mamat and A. Fitri Yusop, Appl. Energy, 2014, 114, 865–873 CrossRef CAS PubMed.
- S. Vedharaj, R. Vallinayagam, W. M. Yang, S. K. Chou and P. S. Lee, Appl. Energy, 2014, 136, 1166–1173 CrossRef CAS PubMed.
- K. Anand, R. P. Sharma and P. S. Mehta, Biomass Bioenergy, 2011, 35, 533–541 CrossRef CAS PubMed.
- K. J. Harrington, Biomass, 1986, 9, 1–17 CrossRef CAS.
- P. S. Mehta and K. Anand, Energy Fuels, 2009, 23, 3893–3898 CrossRef CAS.
- M. Lapuerta, J. Rodríguez-Fernández and O. Armas, Chem. Phys. Lipids, 2010, 163, 720–727 CrossRef CAS PubMed.
- G. Knothe, A. C. Matheaus and T. W. Ryan III, Fuel, 2003, 82, 971–975 CrossRef CAS.
- T. H. Gouw and J. C. Vlugter, J. Am. Oil Chem. Soc., 1964, 41, 524–526 CrossRef CAS.
- P. Hellier, N. Ladommatos and T. Yusaf, Fuel, 2015, 143, 131–143 CrossRef CAS PubMed.
- M. J. Ramos, C. M. Fernández, A. Casas, L. Rodríguez and Á. Pérez, Bioresour. Technol., 2009, 100, 261–268 CrossRef CAS PubMed.
- D. C. Boffito, C. Pirola, F. Galli, A. Di Michele and C. L. Bianchi, Fuel, 2013, 108, 612–619 CrossRef CAS PubMed.
- A. E. Atabani, A. S. Silitonga, H. C. Ong, T. M. I. Mahlia, H. H. Masjuki, I. A. Badruddin and H. Fayaz, Renewable Sustainable Energy Rev., 2013, 18, 211–245 CrossRef CAS PubMed.
- K. Warner, P. Orr and M. Glynn, J. Am. Oil Chem. Soc., 1997, 74, 347–356 CrossRef CAS.
- https://en.wikipedia.org/wiki/Vegetable_oil#cite_note-USDA_ndbc.
- H. An, W. M. Yang and J. Li, Appl. Energy, 2015, 143, 176–188 CrossRef CAS PubMed.
- G. Karavalakis, S. Stournas and E. Bakeas, Sci. Total Environ., 2009, 407, 3338–3346 CrossRef CAS PubMed.
- X. Shi, X. Pang, Y. Mu, H. He, S. Shuai, J. Wang, H. Chen and R. Li, Atmos. Environ., 2006, 40, 2567–2574 CrossRef CAS PubMed.
- S. H. Park, I. M. Youn and C. S. Lee, Fuel, 2011, 90, 748–755 CrossRef CAS PubMed.
- S. Puhan, R. Jegan, K. Balasubbramanian and G. Nagarajan, Renewable Energy, 2009, 34, 1227–1233 CrossRef CAS PubMed.
- J. Narayana Reddy and A. Ramesh, Renewable Energy, 2006, 31, 1994–2016 CrossRef PubMed.
- F. Lujaji, L. Kristóf, A. Bereczky and M. Mbarawa, Fuel, 2011, 90, 505–510 CrossRef CAS PubMed.
- D. C. Rakopoulos, C. D. Rakopoulos, R. G. Papagiannakis and D. C. Kyritsis, Fuel, 2011, 90, 1855–1867 CrossRef CAS PubMed.
- B. Bailey, J. Eberhardt, S. Goguen and J. Erwin, Diethyl ether (DEE) as a renewable diesel fuel, SAE Tech. Pap. Ser., 1997 Search PubMed.
- D. C. Rakopoulos, Fuel, 2013, 105, 603–613 CrossRef CAS PubMed.
- S. Imtenan, H. H. Masjuki, M. Varman, M. A. Kalam, M. I. Arbab, H. Sajjad and S. M. Ashrafur Rahman, Energy Convers. Manage., 2014, 83, 149–158 CrossRef CAS PubMed.
- E. Sukjit, J. M. Herreros, K. D. Dearn, R. García-Contreras and A. Tsolakis, Energy, 2012, 42, 364–374 CrossRef CAS PubMed.
- J. Hou, Z. Wen, Z. Jiang and X. Qiao, Journal of the Energy Institute, 2014, 87, 102–113 CrossRef CAS PubMed.
- L. Zhu, C. S. Cheung, W. G. Zhang and Z. Huang, Fuel, 2011, 90, 1743–1750 CrossRef CAS PubMed.
- G. J. Suppes and M. A. Dasari, Ind. Eng. Chem. Res., 2003, 42, 5042–5053 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |
























