Laser-induced fabrication of Ag@SiO2@Ag sandwich nanostructures having enhanced catalytic performances†
Jaewon Lee and
Du-Jeon Jang*
Department of Chemistry, Seoul National University, NS60, Seoul 151-742, Korea. E-mail: djjang@snu.ac.kr; Fax: +82 2 875 6624; Tel: +82 2 880 4368
First published on 22nd July 2015
Abstract
Ag@SiO2@Ag sandwich nanostructures with highly enhanced catalytic performances have been fabricated in a facile and eco-friendly manner by just irradiating 355 nm laser pulses to an ethanol colloidal solution of Ag@SiO2@Ag-seed nanoparticles for 30 min. The sandwich nanostructures can be further transformed into hollow SiO2@Ag nanostructures with irradiation for an additional 30 min.
Nanocatalysts have attracted considerable attention due to their excellent physical–chemical characteristics along with the rapid development of industry and nanotechnology, and they have been primarily applied in the treatment of waste water containing dye stuffs.1,2 Among diverse nanocatalysts, nanocatalysts based on noble metals such as gold and silver have been intensively investigated because they exhibit unique catalytic properties owing to their great surface-to-volume ratios and their excellent adsorption of reactants.3,4 However, the limitation of noble metal-based nanocatalysts is that they usually tend to aggregate to each other and dissolve as ionic states during catalytic reactions in order to lower the high surface energy, resulting in the significant decrease of the catalytic efficiency.5,6 Because there are also some crucial obstacles like low noble metal-utilization efficiency and high cost, some ways should be found to reduce the amount of noble metals used in specific applications by increasing the catalytic efficiency so as to lower the overall cost. Thus, numerous strategies have been reported recently, such as depositing metallic nanoparticles on silica or polystyrene and coating them with metal oxides.7
Typically, core/shell composite nanostructures with a dielectric solid sphere covered by metallic nanoparticles have been the current focus of research as they have great potential applications in chemical and biological sensors and SERS-based analytical devices and could be reusable as catalysts with enhanced stability.8 However, the utilization of metallic nanoshells suffers from major fabrication drawbacks because their chemical synthesis requires a complex and time-consuming endeavor. The general synthetic processes are as follows:9 (a) fabrication of silica, (b) surface modification of silica with functional groups such as amine or thiol groups, (c) synthesis of small-sized (2–5 nm) seeds, (d) attachment of the seeds to the surface of silica, and (e) growth of the metallic seeds to nanoparticles in a growth solution. The above synthetic methods have also caused substantial environmental problems since many organic/inorganic reagents have been used and discarded in water to fabricate the nanocomposites. Therefore, it is desirable to focus on the development of facilely and eco-friendly synthetic strategies for silica-core metal-shell nanostructures having controlled morphologies and enhanced catalytic properties.
A recent alternative approach for the preparation of metallic nanoparticles is simple laser irradiation to a metallic salt solution without employing any reducing agents.10–14 This method has also been called a photochemical synthesis of metal nanoparticles, which easily allows the control of sizes and morphologies. The main advantages of the photochemical synthesis are as follows:10 the method is clean and convenient because chemical reducing reagents are not required; the formation of nanoparticles can be controlled easily by adjusting laser irradiation; the method can be carried out in diverse media such as surfactant micelles, polymer films, and glasses. In addition, the laser irradiation technique could be exploited as a powerful tool for the controlled reshaping and resizing of wet-chemically produced nanoparticles via the melting, fragmentation, and vaporization of metal nanoparticles with the thermalized photon energy of surface plasmon resonances in general because metallic nanoparticles show depression in melting and boiling temperatures and thermal conductivity with a decrease in their sizes.15–20 Thus, the fabrication and modification of nanomaterials in liquids based on laser irradiation have recently become a rapidly growing field because, compared to other chemical methods, laser-induced fabrication is a “simple” and “green” technique that normally operates in both aqueous and nonaqueous solutions under ambient conditions.
In this communication, we report that upon irradiation of laser pulses, silver seeds-adsorbed Ag-core/SiO2-shell (Ag@SiO2@Ag-seed) nanospheres are transformed to Ag-core/SiO2-shell/Ag-shell (Ag@SiO2@Ag) sandwich nanostructures eco-friendly without employing any reducing agents or linker molecules for surface modification (Fig. 1). The produced sandwich nanostructures show five times enhanced catalytic performances than the pristine nanostructures, indicating that the catalytic properties, as well as the morphologies, of nanoparticles can be controlled facilely by laser irradiation. The silver core of a Ag@SiO2@Ag sandwich nanostructure can be excavated by further laser irradiation to form a hollow SiO2@Ag nanostructure as seen in Fig. 1. The thermalized photon energy of surface plasmon resonances induces the silver core to melt and soak out of the silica surface.
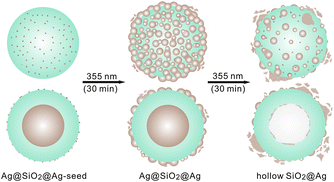 | ||
| Fig. 1 Illustration of processes taking place in a Ag@SiO2@Ag-seed nanoparticle during irradiation with 355 nm laser pulses of 6 ns. The brown indicates silver while the blue indicates silica. | ||
The transmission electron microscopy (TEM) and the high-resolution TEM (HRTEM) images of Fig. 2 show that Ag@SiO2@Ag-seed nanoparticles have undergone shape transformation during irradiation of 355 nm laser pulses to form Ag@SiO2@Ag sandwich nanostructures, which have been subsequently converted into hollow SiO2@Ag nanostructures. The Ag@SiO2@Ag-seed nanoparticles of Fig. 2a, synthesized readily using a polyol process and the StÖber method,21 show that the average diameter of the typical core silver nanospheres, the typical thickness of the silica shells, and the average diameter of silver seeds are 79, 34, and 4 nm (Table 1), respectively. As a colloidal solution of PVP-stabilized silver nanoparticles was added into an ethanol solution containing TEOS, ammonia, and water, ammonia acting as the catalyst hydrolyzed TEOS to form silica shells on silver nanoparticles. On the other hand, Ag+ and ammonia formed Ag(NH3)2+ complex ions, which could be attracted to partially negative-charged silica shells. PVP, acting as a gently reducing and protective agent, has reduced Ag(NH3)2+ ions to silver atoms on silica shells at room temperature, finally producing Ag@SiO2@Ag-seed nanostructures.21 Silver seeds produced on silica surfaces have been served as nucleation sites for the growth of outer silver shells.22 With irradiation of 355 nm laser pulses for 15 min (Fig. S1a†) and 30 min (Fig. 2b), the average diameters of silver seeds adsorbed on the silica surfaces of Ag@SiO2 nanostructures have increased as 10 and 13 nm, respectively, while the diameters of the core silver nanospheres and the thickness values of the silica shells have remained almost invariant. The growth of silver nanoparticles on the silica shells upon laser irradiation to an aqueous colloidal solution containing Ag+ ions in the absence of any reducing agents can be attributed to the photolysis of water, reducing silver ions to their zero valence state through the following scheme:10–14,22,23
| Nanocatalyst | Irradiation time (min) | ds (nm) | λmax (nm) | Rate constant (min−1) |
|---|---|---|---|---|
| a Wavelength at the maximum of surface-plasmon resonances.b Average diameter of silver seeds on silica surfaces.c The value of the rate constant in the absence of any nanocatalysts is 0.0066 min−1. | ||||
| Ag@SiO2@Ag-seed | 0 | 4 | 467 ± 168 | 0.011c |
| 15 | 10 | 470 ± 199 | 0.029 | |
| Ag@SiO2@Ag | 30 | 13 | 477 ± 221 | 0.056 |
| 45 | 8 | 444 ± 202 | 0.024 | |
| Hollow SiO2@Ag | 60 | 9 | 419 ± 179 | 0.012 |
Silver atoms produced by the photolysis of water can grow through the addition of either already reduced silver atoms or silver ions followed by reduction. In our case, silver atoms have been attached to silver seeds on the silica shells at close range under stirring vigorously, resulting in the growth of silver nanoparticles.9 Fig. S1a† shows that silver clusters of 7 nm in an average diameter have also been formed in the colloidal solution upon irradiation of laser pulses for 15 min. It is suggested that the colloidal silver clusters have been adsorbed finally to silver nanoparticles on the silica shells to form Ag@SiO2@Ag sandwich nanostructures during 30 min of laser irradiation.
 | (1) |
| nAg0 → (Ag0)n | (2) |
Fig. S1b† and 2c indicate that laser-chemically produced Ag@SiO2@Ag sandwich nanostructures can be converted into hollow SiO2@Ag nanostructures upon a further irradiation of 355 nm laser pulses. While about 40% of nanostructures have been found to be hollow SiO2@Ag nanostructures upon irradiation for 45 min (Fig. S1b†), almost entire nanostructures produced with irradiation for 1 h have been found to be the hollow structures. A close examination reveals that the average cavity diameter of hollow SiO2@Ag nanostructures is almost the same as the average silver core diameter of initially employed Ag@SiO2@Ag-seed nanoparticles. This indicates that laser irradiation has excavated the silver cores of Ag@SiO2@Ag sandwich nanostructures completely without deteriorating the silica shells. The thermalized photon energy of surface-plasmon resonances is suggested to melt and vaporize the core silver, forming hollow SiO2@Ag nanostructures.19 On the other hand, the average diameter (9 nm) of silver seeds on the silica surfaces in Fig. 2c is substantially smaller than that (13 nm) in Fig. 2b. This suggests that although the sizes of silver seeds on silica surfaces have been increased with irradiation in the beginning, they have been decreased with a further laser irradiation; silver nanoparticles of large sizes on silica surfaces have been fragmented into smaller nanoparticles by a further irradiation as charges accumulated in photoejected silver seeds have repulsed each other due to their mutual electrostatic interactions.20
The time-dependent energy-dispersive X-ray (EDX) elemental profiles of a Ag@SiO2@Ag-seed nanoparticle, scanned along the indicated solid lines of the insetted scanning transmission electron microscopy (STEM) images, in Fig. 3 reveal that while the core silver of the nanostructure has expanded by 15% in diameter for 30 min of laser irradiation, it has soaked out of the silica shell completely during 1 h of laser irradiation to form a hollow SiO2@Ag nanostructure. Fig. 3 displays that silver seeds on the silica shell have grown up to larger silver nanoparticles in 30 min of laser irradiation, forming a Ag@SiO2@Ag sandwich nanostructure. Thus, Fig. 2 and 3 have suggested that the morphologies of metal/semiconductor composite nanostructures can be controlled readily and eco-friendly by adjusting laser irradiation times. The STEM images and the elemental maps of Fig. S2† also reveal that without deteriorating the silica shell, the irradiation of 355 nm laser pulses has converted a Ag@SiO2@Ag-seed nanoparticle into a Ag@SiO2@Ag sandwich nanostructure in 30 min, which has been subsequently transformed into a hollow SiO2@Ag nanostructure in 1 h. In particular, the elemental intensity of silver on the silica shell in Fig. S2b† is much stronger than that in Fig. S2a,† suggesting as well that Ag@SiO2@Ag sandwich nanostructures having enhanced catalytic performances (see below) can be fabricated facilely by irradiating laser pulses to Ag@SiO2@Ag-seed nanoparticles. Fig. S3† displays too that the molar percentage of silver has increased from 48.1% in the Ag@SiO2@Ag-seed nanoparticle to 59.9% in the Ag@SiO2@Ag sandwich nanostructure. On the other hand, the molar percentage of silver in the hollow SiO2@Ag nanostructure has been found to be as small as 19.2%. Together with Fig. 2 and 3, S2 and S3† have demonstrated that the compositions, as well as the structures, of silver-based nanocatalysts can be manipulated eco-friendly by just tuning laser irradiation times without employing any additional chemical reagents.
Fig. 4 and Table 1 present that the surface-plasmon resonances of a Ag@SiO2@Ag-seed colloidal solution change gradually with the irradiation time of 355 nm laser pulses. During the first 30 min of irradiation, the absorption spectrum of the surface-plasmon resonances of silver has shifted to the red by 10 nm and its full width at the half maximum has become wider by 53 nm.10 As discussed already with Fig. 2 and 3, the sizes and coverages of silver seeds on silica surfaces have increased during the first 30 min of laser irradiation to bring about the red shift and broadening of the surface-plasmon resonances of silver.7–9 We consider that increased dipole–dipole interactions among silver nanoparticles grown largely on silica surfaces during laser irradiation have resulted in the coupling of surface-plasmon modes to show the extensive widening and lowering of the absorption spectrum. Thus, Fig. 4 supports that Ag@SiO2@Ag-seed nanoparticles in ethanol have been transformed well into Ag@SiO2@Ag sandwich nanostructures by irradiation of 355 nm laser pulses for 30 min. However, the absorption spectrum of the nanostructures fabricated in the first 30 min of laser irradiation has then shifted largely to the blue by 58 nm with a significant spectral narrowing of 42 nm during the additional 30 min of laser irradiation. The huge blue shift of the absorption spectrum has been attributed to the disappearance of the large core silver nanoparticles due to the formation of hollow SiO2@Ag nanostructures while the significant narrowing of the absorption spectrum has been considered to arise from the photofragmentation of silver nanoparticles on the silica surfaces of Ag@SiO2@Ag sandwich nanostructures.19 The spectral changes of silver-based nanocatalysts in Fig. 4 agree well with their structural changes in Fig. 2 and 3 with the irradiation time of laser pulses. Overall, we assert again that the morphologies of silver-based nanocatalysts can be controlled well by irradiating laser pulses only.
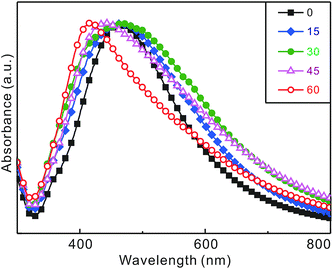 | ||
| Fig. 4 Peak-normalized absorption spectra of Ag@SiO2@Ag-seed nanoparticles dispersed in ethanol after irradiation with 355 nm laser pulses for times indicated in the units of min. | ||
We have evaluated the catalytic properties of silver-based nanocatalysts produced during different irradiation periods of laser pulses by monitoring the time-dependent absorbance changes of RhB reduced catalytically via silver-based nanocatalysts in the presence of KBH4 as indicated in Fig. 5, revealing that the catalytic activity of Ag@SiO2@Ag sandwich nanostructures generated by laser irradiation for 30 min is much higher than that of any other silver-based nanocatalysts. Fig. 5 and Table 1 show that the catalytic rate constant (0.056 min−1) of Ag@SiO2@Ag sandwich nanostructures fabricated with laser irradiation for 30 min is larger five times than that (0.011 min−1) of pristine Ag@SiO2@Ag-seed nanoparticles. The catalytic degradation mechanism of RhB via silver-based nanocatalysts in the presence of KBH4 could be explained as follows.24 RhB is electrophilic and BH4− is nucleophilic in comparison with silver-based nanocatalysts, meaning that the nucleophilic BH4− can donate electrons to silver-based nanocatalysts and that the electrophilic RhB can capture electrons from silver-based nanocatalysts. Thus, the silver-based nanocatalysts facilitate electron transfer from BH4− (donor) to the RhB (acceptor) through their catalytic surfaces;7,8 the silver-based nanocatalysts serve as electron relays for the degradation reaction of RhB in the presence of KBH4. Note that although KBH4 is known to be a strong reducing agent of organic molecules, RhB in the presence of KBH4 hardly decomposes in the absence of silver-based nanocatalysts (Fig. 5c and Table 1). The large enhancement of the catalytic activity of silver-based nanostructures with laser irradiation for 30 min has been attributed to the increased sizes and coverages of silver seeds on silica surfaces by irradiation as already presented with Fig. 2–4.16,21,24 It has been reported24 that laser irradiation increases the catalytic performances of hollow platinum nanospheres and silica-coated gold nanorods in the degradation of organic dyes largely via lowering the energy barrier. Thus, we suggest that the increase of the catalytic rate constant by laser irradiation for 30 min is due to decrease in the activation energy of the catalytic reaction on silver nanoparticles grown largely on silica surfaces owing to 355 nm laser pulses; the formation of the activated complex for the catalytic degradation reaction of RhB is considered to be energetically favorable substantially on the metallic surfaces of laser-fabricated Ag@SiO2@Ag sandwich nanostructures.24 Fig. 5c and Table 1 indicate that the catalytic rate constant (0.012 min−1) of hollow SiO2@Ag nanostructures, which have been fabricated with laser irradiation to Ag@SiO2@Ag-seed nanoparticles for 60 min, is much smaller than that (0.056 min−1) of Ag@SiO2@Ag sandwich nanostructures. Although the exact catalytic mechanisms of silver-based nanocatalysts have not been fully explored yet, we suggest that sandwich nanostructures play an important role in the catalytic degradation of RhB in the presence of KBH4. Thus, Fig. 5 and Table 1 have demonstrated that Ag@SiO2@Ag sandwich nanostructures with high catalytic activity can be fabricated facilely by irradiating 355 nm laser pulses to Ag@SiO2@Ag-seed nanoparticles for 30 min.
Conclusions
In summary, upon irradiation of 355 nm laser pulses for 30 min silver seeds adsorbed on the silica surfaces of Ag@SiO2@Ag-seed nanoparticles have grown up to larger silver nanoparticles successfully via the photochemical reduction of silver ions, finally forming Ag@SiO2@Ag sandwich nanostructures with highly enhanced catalytic performances; the catalytic activity of laser-fabricated Ag@SiO2@Ag sandwich nanostructures for the degradation reaction of RhB in the presence of KBH4 is higher five times than that of unirradiated Ag@SiO2@Ag-seed nanoparticles. Thus, this laser-induced synthetic method of silver-based nanocatalysts can be regarded as a new approach of green chemistry because it does not require any reducing agents nor any surface-treatment processes. We have also shown that the sandwich nanostructures can be transformed into hollow SiO2@Ag nanostructures with a further irradiation of laser pulses for 30 min. Overall, we suggest that the catalytic performances, as well as the morphologies and the compositions, of silver-based nanocatalysts can be controlled facilely and eco-friendly by adjusting the irradiation times of 355 nm laser pulses.Acknowledgements
This work was supported by research grants through the National Research Foundation (NRF) of Korea funded by the Korea government (2012-006345 and 2014-057382). D.J.J. is also thankful to the SRC program of NRF (2007-0056095).Notes and references
- A. T. Bell, Science, 2003, 299, 1688 CrossRef CAS PubMed.
- K. Dong, Z. Liu and J. Ren, CrystEngComm, 2013, 15, 6329 RSC.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS PubMed.
- K. M. Yeo, S. Choi, R. M. Anisur, J. Kim and I. S. Lee, Angew. Chem., Int. Ed., 2011, 50, 745 CrossRef CAS PubMed.
- J. Zeng, Q. Zhang, J. Chen and Y. Xia, Nano Lett., 2010, 10, 30 CrossRef CAS PubMed.
- M. A. Mahmoud, F. Saira and M. A. El-Sayed, Nano Lett., 2010, 10, 3764 CrossRef CAS PubMed.
- J. Lee, K. Han and D.-J. Jang, Appl. Catal., A, 2014, 380, 469 Search PubMed; Y. Kim and D.-J. Jang, Chem. Commun., 2013, 49, 8940 RSC; K. Wang, X. Zhang, C. Niu and Y. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 1272 Search PubMed; J. Zhang, J. Liu, S. Wang, P. Zhan, Z. Wang and N. Ming, Adv. Funct. Mater., 2004, 14, 1089 CrossRef CAS PubMed; Y. Li, Y. Wu, Y. Gao, S. Sha, J. Hao, G. Cao and C. Yang, RSC Adv., 2013, 3, 26361 RSC; G. Li and Z. Tang, Nanoscale, 2014, 6, 3995 RSC.
- J.-A. Kwak, D. K. Lee and D.-J. Jang, Appl. Catal., B, 2013, 142, 323 CrossRef CAS PubMed; L. You, Y. Mao and J. Ge, J. Phys. Chem. C, 2012, 116, 10753 Search PubMed; Z. Deng, H. Zhu, B. Peng, H. Chen, Y. Sun, X. Gang, P. Jin and J. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 5625 Search PubMed; Z. Deng, M. Chen and L. Wu, J. Phys. Chem. C, 2007, 111, 11692 Search PubMed.
- Z.-J. Jiang and C.-Y. Liu, J. Phys. Chem. B, 2003, 107, 12411 CrossRef CAS; W. Cai, W. Wang, Y. Yang, G. Ren and T. Chen, RSC Adv., 2014, 4, 2295 RSC; J.-W. Choi, H. Kang, M. Lee, J. S. Kang, S. Kyeong, J.-K. Yang, J. Kim, D. H. Jeong, Y.-S. Lee and Y.-E. Sung, RSC Adv., 2014, 4, 19851 RSC; A. M. Brito-Silva, R. G. Sobral-Filho, R. Babosa-Silva, C. B. de Araujo, A. Galembeck and A. G. Brolo, Langmuir, 2013, 29, 4366 CrossRef PubMed.
- K. E. Peceros, X. Xu, S. R. Bulcock and M. B. Cortie, J. Phys. Chem. B, 2005, 109, 21516 CrossRef CAS PubMed.
- H. Zeng, X.-W. Du, S. C. Singh, S. A. Kulinich, S. Yang, J. He and W. Cai, Adv. Funct. Mater., 2012, 22, 1333 CrossRef CAS PubMed; M. Sakamoto, M. Fujistuka and T. Majima, J. Photochem. Photobiol., C, 2009, 10, 33 CrossRef PubMed.
- V. Amendola and M. Meneghetti, Phys. Chem. Chem. Phys., 2013, 15, 3027 RSC.
- J. P. Abid, A. W. Wark, P. F. Breve and H. H. Girault, Chem. Commun., 2002, 792 RSC.
- H. S. Shin, H. J. Yang, S. B. Kim and M. S. Lee, J. Colloid Interface Sci., 2004, 274, 89 CrossRef CAS PubMed; H. H. Huang, X. P. Ni, G. L. Loy, C. H. Chew, K. L. Tan, F. C. Loh, J. F. Deng and G. Q. Xu, Langmuir, 1996, 12, 909 CrossRef; A. Henglein, Chem. Mater., 1998, 10, 444 CrossRef; W. Wang and A. Asher, J. Am. Chem. Soc., 2001, 123, 12528 CrossRef PubMed.
- D. Werner and S. Hashimoto, Langmuir, 2013, 29, 1295 CrossRef CAS PubMed; A. Takami, H. Kurita and S. Koda, J. Phys. Chem. B, 1999, 103, 1226 CrossRef.
- M. R. Kim, J.-Y. Kim, S. J. Kim and D.-J. Jang, Appl. Catal., A, 2011, 393, 317 CrossRef CAS PubMed.
- S. Kundu and H. Liang, Langmuir, 2010, 26, 6720 CrossRef CAS PubMed; C. M. Aguirre, C. E. Moran, J. F. Young and N. J. Halas, J. Phys. Chem. B, 2004, 108, 7040 CrossRef; D. Werner, S. Hashimoto and T. Uwada, Langmuir, 2010, 26, 9956 CrossRef PubMed.
- R. Jin, Y. C. Cao, E. Hao, G. S. Metraux, G. C. Schatz and C. A. Mirkin, Nature, 2003, 425, 487 CrossRef CAS PubMed; A. Callegari, D. Tonti and M. Chergui, Nano Lett., 2003, 3, 1565 CrossRef; R. Jin, Y. Cao, C. A. Mirkin, K. L. Kelly, G. C. Schatz and J. G. Zheng, Science, 2001, 294, 1901 CrossRef PubMed.
- S. J. Kim, C. S. Ah and D.-J. Jang, Adv. Mater., 2007, 19, 1064 CrossRef CAS PubMed; C. S. Ah, S. J. Kim and D.-J. Jang, J. Phys. Chem. B, 2006, 110, 5486 CrossRef PubMed.
- P. V. Kamat, J. Phys. Chem. B, 2002, 106, 7729 CrossRef CAS; P. V. Kamat, M. Flumiani and G. V. Hartland, J. Phys. Chem. B, 1998, 102, 3123 CrossRef; S. Inasawa, M. Sugiyama and Y. Yamaguchi, J. Phys. Chem. B, 2005, 109, 3104 CrossRef PubMed; J. H. Hodak, A. Henglein, M. Giersig and G. V. Hartland, J. Phys. Chem. B, 2000, 104, 11708 CrossRef.
- C. Li, J. Mei, S. Li, N. Lu, L. Wang, B. Chen and W. Dong, Nanotechnology, 2010, 21, 245602 CrossRef PubMed.
- T. Liu, D. Li, D. Yang and M. Jiang, Colloids Surf., A, 2011, 387, 17 CrossRef CAS PubMed; H. H. Park, K. Woo and J.-P. Ahn, Sci. Rep., 2013, 3, 1497 Search PubMed.
- J. Belloni, M. Mostafavi, H. Remita, J.-L. Marigneir and M.-O. Delcourt, New J. Chem., 1998, 1239 RSC; C. L. Thomsen, D. Madsen, S. R. Keiding and J. Thogersen, J. Chem. Phys., 1999, 110, 3453 CrossRef CAS PubMed; V. G. Pol, D. N. Srivastava, O. Palchik, V. Palchik, M. A. Slifkin, A. M. Weiss and A. Gedanken, Langmuir, 2002, 18, 3352 CrossRef; A. S. Nikolov, R. G. Nikov, I. G. Dimitrov, N. N. Nedyalkov, P. A. Atanasov, M. T. Alexandrov and D. B. Karashanova, Appl. Surf. Sci., 2013, 280, 55 CrossRef PubMed.
- H. Lee, J.-A. Kwak and D.-J. Jang, J. Phys. Chem. C, 2014, 118, 22792 Search PubMed; M. Son, J. Lee and D.-J. Jang, J. Mol. Catal. A: Chem., 2014, 385, 38 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, three additional figures. See DOI: 10.1039/c5ra09519k |
| This journal is © The Royal Society of Chemistry 2015 |



