Superior methanol electrooxidation activity and CO tolerance of mesoporous helical nanospindle-like CeO2 modified Pt/C†
Jing Chen,
Songmei Li*,
Juan Du,
Jianhua Liu,
Mei Yu,
Shiming Meng and
Bo Wang
Key Laboratory of Aerospace Advanced Materials and Performance of Ministry of Education, School of Materials Science and Engineering, Beihang University, Beijing, 100191, P. R. China. E-mail: songmei_li@buaa.edu.cn; Tel: +86 10 82317103
First published on 10th July 2015
Abstract
In an attempt to enhance the electrocatalytic activity and CO tolerance of ceria modified Pt/C electrodes, a novel structured ceria material has been developed. Left-handed helical CeO2 nano-spindles with mesoporous structures were successfully synthesized through a template-free based precursor method on a large scale. By a microwave-assisted polyol synthesis process, the ceria modified Pt/C electrocatalysts were synthesized. The helical CeO2 nanospindle based electrode Pt@Heli-CeO2/C exhibits superior electrochemically active surface areas, and significantly enhanced methanol oxidation catalytic activity and CO antipoisoning activity, compared to Pt/C and a nano-octahedral CeO2 modified electrode Pt@Octa-CeO2/C. The experimental results show that Pt@Heli-CeO2/C possesses 8.2 times and 3.2 times higher activity for methanol electrooxidation than Pt/C and Pt@Octa-CeO2/C, respectively. This remarkable enhancement could be attributed to the reasons as follows: compared to octahedral CeO2, the unique helical CeO2 is more conductive with better electron transfer and can provide more active surface sites to strengthen the support–metal interactions based on an electronic transfer mechanism from CeO2 to Pt, thus helical CeO2 can promote better dispersion of the Pt(0) nanocrystallites and a high concentration of metallic Pt(0) in the composition.
Introduction
Direct methanol fuel cells (DMFCs) have been attracting attention as promising power storage devices for electric vehicle and portable power applications for decades,1–3 due to their unique properties, such as their low operating temperature, low emission of pollutants and relatively high energy density.4 However, intermediates during the anodic oxidation processes, especially carbon monoxide, will poison a platinum based catalyst and lead to a low methanol oxidation activity at the anode. To mitigate the CO poisoning, one solution is to design Pt-based bimetallic or trimetallic catalysts, a number of multi-alloyed electrocatalysts, such as PtSn,5,6 PtRu,7,8 PtRh,9,10 PtCu,11 PtRuIr,12 and PtRuNi13 have been previously fabricated. Another strategy is to use transition metal oxides supported on a modified electrocatalyst, such as Pt/SnO2,14 Pt/TiO2,15,16 and Pt/SiO2,17 based on a multi-functional mechanism18 and electronic modification.19In particular, CeO2 is considered to be a potential co-catalyst because of its high oxygen storage capacity and anticorrosion ability in acidic media, especially for alcohol oxidation. It was reported that ceria modified Pt/C electrocatalysts exhibited enhanced methanol oxidation activity compared with that of Pt/C20 or PtRu/C.21,22 However, the low electron transfer rate of CeO2 together with the weak interaction between CeO2 and Pt, still limits its catalytic performance. Until now, many efforts have focused on the structure designing and doping of CeO2-modified electrocatalysts to improve the synergistic effect and electronic conductivity. Du et al.23 developed a Pd-surrounded-CeO2−x catalyst via a three-phase-transfer approach, and it exhibited an excellent catalytic performance for methanol oxidation attributed to the enhanced interfacial interaction between CeO2−x and Pd. Chu24 and co-workers synthesized a nitrogen doped carbon packed Pt/CeO2 catalyst via the in situ carbonization of an ionic liquid, and it exhibited greatly enhanced electrical conductivity of the CeO2. But fewer papers have focused on a new structure design of CeO2 to enhance the electron conduction. It was found that, the configuration of the metal oxide played a critical role in the electronic structure16,25,26 and the synergistic interaction between the metal oxide and Pt.4,27 Therefore, it is useful to explore the novel morphology of CeO2 as a cocatalyst in methanol electrooxidation.
Helical structured materials, which exhibit sophisticated morphology as well as unique chemical and physical activity, are usually associated with biological polymers or organic materials.28 Inorganic materials with helical nanostructures have not been widely explored.
In this investigation, when L-AspNa was used as an additive, a uniform helical spindle-like cerium compound was successfully fabricated through the interaction among the additives (L-AspNa), precipitating agent (Na2CO3), and inorganic source (Ce(NO3)3·6H2O). After a thermal decomposition, the mesoporous nanospindle-like CeO2 polycrystal with a helical architecture was produced. This helical nanospindle-like CeO2 based catalyst displayed an 8.2 fold enhancement of methanol oxidation performance compared to Pt/C.
Experimental section
Synthesis of ceria nanostructures
All chemicals used were purchased from Chemical CO., LTD. directly. In the synthesis of the helical CeO2, 2 mmol Ce(NO3)3·6H2O was dissolved in a 10 mL 0.6 M AspNa solution, then 10 mL of 0.15 M Na2CO3 was dropped into the solution and was stirred vigorously at ambient conditions for 3 h. After that, the resulting white turbid liquid was poured into a 30 mL Teflon-lined stainless autoclave and was treated at 160 °C for 24 h. The obtained products were washed with deionized water and ethanol twice, then stayed overnight at 60 °C in air, and were named as Heli-CeO2-P. Then Heli-CeO2-P was calcined under air conditions at 350 °C for 3 h, to get the helical structured ceria (named as Heli-CeO2).For the preparation of the CeO2 nano-octahedron (Octa-CeO2), all the experimental procedures remained the same as described above, except for the absence of amino acids and further calcination.
Characterization
The X-ray diffraction (XRD) data were recorded by a Rikagu D/max 2200PC, with Cu Kα radiation (λ = 1.5406 Å). The thermogravimetric-differential thermal (TG-DTA) curves were obtained on a NETZSCH instrument DSC/TGA-STA 449F3 in air at a heating rate of 10 °C min−1, using α-Al2O3 as a reference. The field-emission scanning electron microscopy (FESEM) observations were performed by a FEI SIRION microscope. The transmission electron microscopy (TEM), high-resolution TEM (HRTEM), and selected-area electron diffraction (SAED) data were collected on a JEOL 2010 FEG microscope at 200 kV. The N2 adsorption–desorption isotherms and Brunauer–Emmett–Teller (BET) surface areas of the nanostructured ceria samples were recorded on a BELSORP-mini II sorption instrument at 77 K. The H2-temperature-programmed reduction (TPR) was performed on a Quantachrome ChemBET 3000 analyzer at a rate of 10 °C min−1 using 10% H2/Ar mixed gas with a flow of 25 mL min−1. The X-ray photoelectron spectroscopy (XPS) was determined using a PHI-Quantera SXM.Preparation of electrocatalyst composites
The electrocatalysts were prepared as reported in the literature.29 Firstly, a liquid mixture of ethylene glycol (EG) and isopropyl alcohol with a volume ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was prepared. Secondly, 5 mg CeO2 and 20 mg carbon powder were dispersed into the solution with ultrasonication until a homogeneous mixture was formed. After adding 5.85 mL of 0.01 M H2PtCl6–EG into the mixture, 0.8 M KOH–EG was used to adjust the pH to 10 dropwise. After stirring for another 6 h, the resultant mixture was heated in a domestic microwave oven (750 W, Galanz, China) for 50 s. After cooling to room temperature, 15 mL of 0.1 M HNO3 was dropped in to adjust the pH to ∼4 with vigorous stirring for 12 h, the resultant black suspension was centrifuged and washed with distilled water and ethanol twice. The product was then dried at 40 °C for 12 h in a vacuum drying oven. To evaluate the role of ceria, a Pt/C electrocatalyst was also synthesized using a similar procedure, but without CeO2. It was very easy to reproduce Pt nanoparticles by this microwave method. The resultant catalysts were named as Pt@Heli-CeO2/C-re1, Pt@Heli-CeO2/C-re2, Pt@Octa-CeO2/C-re1, Pt@Octa-CeO2/C-re2, Pt/C-re1 and Pt/C-re2, the results are shown in the ESI.†
1 was prepared. Secondly, 5 mg CeO2 and 20 mg carbon powder were dispersed into the solution with ultrasonication until a homogeneous mixture was formed. After adding 5.85 mL of 0.01 M H2PtCl6–EG into the mixture, 0.8 M KOH–EG was used to adjust the pH to 10 dropwise. After stirring for another 6 h, the resultant mixture was heated in a domestic microwave oven (750 W, Galanz, China) for 50 s. After cooling to room temperature, 15 mL of 0.1 M HNO3 was dropped in to adjust the pH to ∼4 with vigorous stirring for 12 h, the resultant black suspension was centrifuged and washed with distilled water and ethanol twice. The product was then dried at 40 °C for 12 h in a vacuum drying oven. To evaluate the role of ceria, a Pt/C electrocatalyst was also synthesized using a similar procedure, but without CeO2. It was very easy to reproduce Pt nanoparticles by this microwave method. The resultant catalysts were named as Pt@Heli-CeO2/C-re1, Pt@Heli-CeO2/C-re2, Pt@Octa-CeO2/C-re1, Pt@Octa-CeO2/C-re2, Pt/C-re1 and Pt/C-re2, the results are shown in the ESI.†
Electrochemical measurements
The electrochemical measurements were all tested on a Princeton VMC-4 potentiostation with a conventional three-electrode system (working electrode: glassy-carbon electrode (GCE) with a diameter of 6 mm, reference electrode: an Ag/AgCl electrode, and counter electrode: Pt wire) at room temperature. 5 mg of the Pt/C, Pt@Heli-CeO2/C or Pt@Octa-CeO2/C composite powders, was re-dispersed in 1.0 mL of isopropanol under sonication, then 0.1 mL of a Nafion solution (0.5 wt%) was added to form the catalytic ink. To prepare the working electrode, 10 μL of the catalyst ink was dropped on a polished mirror GCE using a pipette and was dried at room temperature. Prior to the electrochemical measurements, the electrolytes were deoxygenated with N2 for 30 min.The cyclic voltammetry (CV) was recorded in a 3 mL 0.5 M H2SO4 and 3 mL 2 M CH3OH solution, from 0 to 1.0 V at 50 mV s−1 until a steady CV was obtained. The chronoamperometry (CA) measurements were carried out at 0.5 V for 3600 s in 0.5 M H2SO4 and 2 M CH3OH. The CO stripping voltammetry and electrochemical active surface area (ECSA) measurements of platinum were performed in 0.5 M H2SO4 at 20 mV s−1. Prior to the CO adsorption, the system was deoxygenated by bubbling with N2 for 30 min. And then at −0.1 V CO (10% CO in Ar balance) was adsorbed onto the electrodes by bubbling with CO gas for 10 min. To remove any dissolved CO, N2 was used to purge the system for another 15 min. Then the adsorbed CO was oxidized from −0.2 V to 1.1 V by CV. The ECSA of platinum was calculated with the formula ECSApt = QH/(0.21MPt).30–32 The electrochemical impedance spectroscopy (EIS) was performed during frequency range 0.01–100 kHz with 30 points per decade.
Results and discussion
With and without L-AspNa, two products were prepared via a hydrothermal treatment of the Ce(NO3)3 (aq.) and Na2CO3 (aq.) mixture. The morphologies and sizes of these two cerium compounds were investigated by SEM, as shown in Fig. 1. Fig. 1a presents the picture of the cerium precursors synthesized with the help of L-AspNa. Most of the products in this picture present a unique spindle-like morphology. At high magnification (Fig. 1b), a left-handed helical structure, named Heli-CeO2-P, can be observed distinctly. The spiral diameter ranges from 40 to 100 nm. In contrast, if the sample was synthesized without L-AspNa, the compound consists of regular octahedron particles, as shown in Fig. 1c and d. This structure, with a smooth surface and edge length ranging from 100 to 600 nm, is named Octa-CeO2. The SEM results indicate that when L-AspNa is used as an additive, the product morphology can be obviously changed from nano-octahedron to nano-spindle. | ||
| Fig. 1 SEM images of CeO2 with (a and b) helical spindle and (c and d) nano-octahedron morphologies. | ||
The XRD results of the two cerium compounds are exhibited in Fig. 2, the Octa-CeO2 (Fig. 2 blue line) is face-centered cubic CeO2 (JCPDS Card No. 65-5923). The strong diffraction peaks at the Bragg angles of 28.4°, 33.3°, 47.6°, 56.3°, 59.1°, 69.4°, 76.7°, and 79.1° can be assigned to (111), (200), (220), (311), (222), (400), (331), and (420) crystal planes, respectively. However, the Heli-CeO2-P is amorphous from the XRD measurement (Fig. 2, black line). These results imply that, not only the morphology, but also the crystalline process of the precursor was affected by the addition of L-AspNa. Additionally, TG-DTA was performed to confirm the complete decomposition temperature of Heli-CeO2-P. The TG-DTA results (see Fig. S1†) show that 350 °C was the minimal temperature to obtain pure CeO2 from the amorphous precursor. The XRD pattern of the yellowish ceria power obtained after calcinating, named Heli-CeO2, is presented in Fig. 2 (red line). The existence of the narrow and intense diffraction peaks indicates the structure is cubic CeO2.
A closer inspection of the SEM results of Heli-CeO2 (Fig. 3a) shows ceria nanospindles with a fair uniformity. The helical structure was well preserved after calcination. SEM (Fig. 3a and b) and TEM (Fig. 3c) images, suggest the presence of a tip at each end of a CeO2 nanospindle. The length of a Heli-CeO2 nanospindle is approximately 900 nm, and the diameter of the widest part is around 90 nm. A closer examination of one helical spindle-like Heli-CeO2 (Fig. 3d) indicates that nanoparticles, with diameters of about 8 nm, are the building blocks for Heli-CeO2. This was also confirmed by SAED patterns (inset in Fig. 3d) of Heli-CeO2, which show a polycrystalline diffraction pattern. In Fig. 3d, the interplanar spacing of the lattice fringes (111), (200) and (220) were identified to be 0.312, 0.274 and 0.191 nm, respectively. The TEM image in Fig. 3e, confirmed a fairly perfect nano-octahedron morphology with clear edges and corners on Octa-CeO2. The HRTEM image in Fig. 3f displays clear (111) planes with fringe spacings of 0.312 nm. The corresponding SAED pattern (inset in Fig. 3f) shows a typical single crystalline structure of the Octa-CeO2 sample. The N2 adsorption/desorption isotherms (shown in Fig. S2†) confirmed the different porous structures for Heli-CeO2 and Octa-CeO2: while there were mesopores, accumulated by the nanoparticles, within the helical spindle-like structure, a porous structure was not present in Octa-CeO2. Correspondingly, Heli-CeO2 owns a much larger specific surface area (∼27 m2 g−1) than Octa-CeO2 (∼2 m2 g−1). The H2-TPR results reveal that the helical spindle Heli-CeO2 sample shows two highly intense low-temperature reduction peaks at 421 °C and 445 °C, whereas, the nano-octahedron Octa-CeO2 shows one reduction peak at 508 °C. This difference demonstrates that helical spindle Heli-CeO2 is much more reducible and active than the nano-octahedron Octa-CeO2 materials (Fig. S3†).
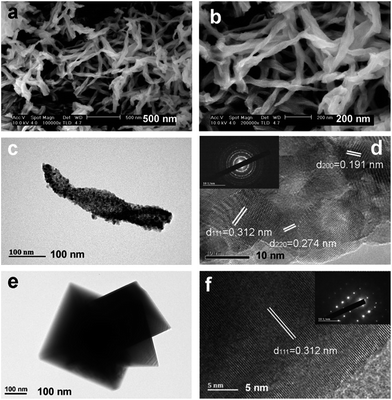 | ||
| Fig. 3 SEM (a and b) and TEM (c and d) images of helical spindle-like CeO2, TEM images (e and f) of nano-octahedron CeO2. | ||
The ceria promoted Pt/C electrocatalysts were prepared by a microwave-assisted polyol synthesis method, and the TEM images of the electrocatalysts are exhibited in Fig. 4. In the case of Pt@Octa-CeO2/C and Pt/C, the distribution of the Pt nanoparticles was not as uniform as for Pt@Heli-CeO2/C, and large Pt clusters were detected on a selected surface of Octa-CeO2 supported carbon black (Fig. 4c) and bare carbon black (Fig. 4e), respectively. On the other hand, the helical spindle-like Heli-CeO2 supported carbon black showed significantly improved dispersion and distribution of the Pt nanoparticles, as presented in Fig. 4a. The average diameter of the Pt nanoparticles with well-defined fingerprints (Fig. 4b, d and f) in Pt@Heli-CeO2/C, Pt@Octa-CeO2/C and Pt/C were about 2.2, 2.2 and 2.5 nm, respectively. The repeated results are shown in Fig. S4.† Helical spindle-like Heli-CeO2, with a high concentration of active crystal faces ((220) and (200)) and oxygen vacancies, offers a stronger metal–support interaction between Heli-CeO2 and the Pt nanoparticles based on electronic transfer mechanisms.18,32,33 Therefore, the surface electronic properties of Pt were modified, attributed to a shift in the d-band center of the surface Pt atoms, to induce a higher concentration of metallic Pt(0). Furthermore, the stronger interaction between the Pt nanoparticles and helical spindle-like Heli-CeO2 can prevent agglomeration of the Pt nanoparticles; forming highly dispersed and stable Pt nanoparticles.
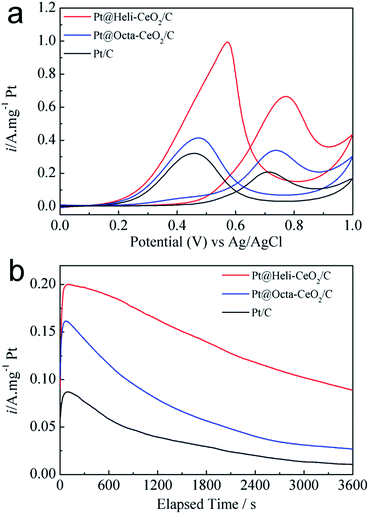
To understand the role of the helical CeO2–H in enhancing the catalyst conductivity, electrochemical impedance spectroscopy (EIS) was carried out. As shown in Fig. 5a, the same pattern appears and the semicircle of Pt@Heli-CeO2/C is much smaller than Pt@Octa-CeO2/C and Pt/C, reflecting that the impedance of the helical ceria modified Pt@Heli-CeO2/C is reduced significantly compared with the octahedron ceria modified Pt@Octa-CeO2/C and Pt/C.33,34 This remarkable enhancement in the electron transmission may be attributed to the helical structure, which is more conductive with better electron transfer than that of the corresponding octahedral structures.35,36 The electrochemical surface characteristics of Pt@Heli-CeO2/C are compared with Pt@Octa-CeO2/C and Pt/C catalysts using cyclic voltammetry (Fig. 5b). As shown by the black curve for the Pt/C electrocatalyst, the platinum oxide formation/reduction peaks are less obvious, which is ascribed to the aggregation of Pt and the corrosion of the carbon powder.37 In contrast, when ceria is added, the Pt oxide formation/reduction peaks are distinctly visible. In addition, the voltammogram of Pt@Octa-CeO2/C exhibits a similar double layer charging current and hydrogen adsorption/desorption peaks to that of the Pt/C catalyst. The similar features of Pt@Octa-CeO2/C and Pt/C indicate that the interaction between octahedron Octa-CeO2 and the Pt nanoparticles in Pt@Octa-CeO2/C is not strong. However, for Pt@Heli-CeO2/C, the hydrogen adsorption/desorption peaks become broader and more noticeable, and the double layer charging current becomes larger, compared with the octahedron ceria modified Pt@Octa-CeO2/C and Pt/C, implying a stronger interfacial interaction between Heli-CeO2 and Pt.38 The ECSA of Pt@Heli-CeO2/C estimated from the hydrogen adsorption peaks is 80.95 m2 g−1, which is almost two times larger than that of Pt@Octa-CeO2/C and Pt/C (47.16 m2 g−1 and 40.67 m2 g−1, respectively). This is attributed to the improvement in electron conductivity and the highly dispersed and multifaceted Pt crystallites on the helical spindle-like Heli-CeO2 promoted Pt/C electrocatalyst.
Fig. 5c presents the COad stripping curves of Pt@Heli-CeO2/C, Pt@Octa-CeO2/C and Pt/C. As shown in Fig. 5c, the CO onset and peak oxidation potentials are 0.5, 0.53 and 0.68 V, and 0.62, 0.64 and 0.72 V for Pt@Heli-CeO2/C, Pt@Octa-CeO2/C and Pt/C, respectively. The comparison indicates that the nano-shaped ceria promoted Pt/C electrocatalysts present lower oxidation potentials and broader COad oxidation peaks, with the helical nanospindle Heli-CeO2 promoted Pt/C showing the highest CO oxidation activity among these three electrocatalysts. The helical morphology of Heli-CeO2, possesses more active crystal facets and oxygen vacancies, which could increase the synergistic interactions and intimate contact between the interface of Pt and the oxygen-containing species of the helical structured Heli-CeO2 due to electronic transfer mechanisms.19 The OH groups generated on the surface of Heli-CeO2, react with the absorbed CO on the Pt particles, and then produce CO2.39
To investigate the interaction between the nanostructured ceria and the platinum nanoparticles in the as-prepared electrocatalyst, XPS spectroscopy was performed to obtain the chemical and electronic information. The Pt 4f and Ce 3d XPS data of Pt@Heli-CeO2/C and Pt@Octa-CeO2/C are shown in Fig. 6, and the deconvoluted results are summarized in Table 1. The Pt 4f spectra are deconvoluted into 5 peaks, including three Pt chemical states: Pt(0), Pt(II), and Pt(IV).40–42 For Pt@Heli-CeO2/C, the Pt 4f binding energies of the Pt(0) peaks show a larger shift to lower energies by about 0.3 eV, whereas the Pt(0) peaks show only a 0.1 eV shift for Pt@Octa-CeO2/C compared to Pt/C, and this can be attributed to the much stronger interaction between Pt and the helical structured Heli-CeO2.39,43,44 Moreover, the metallic Pt species in Pt@Heli-CeO2/C, Pt@Octa-CeO2/C, and Pt/C, is estimated to be 79.1, 62.5 and 76.9%, respectively. The relatively high ratio of metallic Pt will be beneficial for high catalytic performance.40 In addition, the Ce 3d XPS spectra reveal three-lobed envelopes (around 878–895 eV, 895–912 eV and approximately 917 eV) such as those depicted in Fig. 6b and d. The Ce 3d spectra are deconvoluted into 8 peaks.45 The percentage of Ce(III) relative to the total amounts of Ce is estimated to be 40.5 and 21.8% for the Pt@Heli-CeO2/C and Pt@Octa-CeO2/C catalysts, respectively. The higher concentration of Ce(III) in the Heli-CeO2 modified Pt/C catalysts are due to the smaller size of the constituent ceria nanoparticles, which possess a higher proportion of surface defects. The obvious differences in the Ce(III) relative intensities between Pt@Heli-CeO2/C and Pt@Octa-CeO2/C could have a great impact on the reducibility of the Pt species. This is supported by the analysis of the Pt 4f spectrum,46 showing a Pt(0) relative intensity of 79.1 and 62.5% for Pt@Heli-CeO2/C and Pt@Octa-CeO2/C respectively. The XPS data show that the Pt@Heli-CeO2/C catalyst has 40.5% Ce(III) species, which would give rise to a higher oxidation activity; moreover, due to the stronger interaction between the helical structured Heli-CeO2 and Pt particles, many more Pt species can be easily reduced to Pt(0).
 | ||
| Fig. 6 (a) Pt 4f and (b) Ce 3d core-level XPS spectra of Pt@Heli-CeO2/C samples. (c) Pt 4f and (d) Ce 3d core-level XPS spectra of Pt@Octa-CeO2/C samples. | ||
| Sample | Peak | Species | Binding energy (eV) | Relative ratio | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pt/C | Pt 4f | 0 | 71.7 | 75.0 | 76.9 | ||||
| +2 | 73.1 | 76.5 | 18.9 | ||||||
| +4 | 78.3 | 4.2 | |||||||
| Pt@Heli-CeO2/C | Pt 4f | 0 | 71.4 | 74.7 | 79.1 | ||||
| +2 | 72.6 | 76.4 | 16.8 | ||||||
| +4 | 78.3 | 4.1 | |||||||
| Ce 3d | +3 | 886.0 | 904.7 | 40.5 | |||||
| +4 | 882.5 | 889.7 | 898.4 | 901.3 | 908.4 | 916.9 | 59.5 | ||
| Pt@Octa-CeO2/C | Pt 4f | 0 | 71.6 | 74.9 | 62.5 | ||||
| +2 | 72.7 | 76.8 | 33.3 | ||||||
| +4 | 78.2 | 4.2 | |||||||
| Ce 3d | +3 | 886.0 | 905.2 | 21.8 | |||||
| +4 | 882.9 | 888.9 | 898.9 | 901.8 | 908.4 | 917.1 | 78.2 | ||
The voltammetric curves from a N2 saturated 0.5 M H2SO4 and 2.0 M CH3OH aqueous solution are shown in Fig. 7a.47,48 Evidently, the current densities at the maximum in the forward sweep of these electrodes are, in order, Pt@Heli-CeO2/C (0.99 A mg−1 Pt) > Pt@Octa-CeO2/C (0.41 A mg−1 Pt) > Pt/C (0.32 A mg−1 Pt), indicating that the oxidation current density for Pt@Heli-CeO2/C is more than 3.1 times higher than that of Pt/C at the maximum in the forward sweep. The onset potential for the ceria promoted Pt/C electrocatalysts is lower than that of the Pt/C electrocatalyst in the forward sweep of the CV curves, suggesting that the performance of the ceria promoted Pt/C electrocatalysts is superior to Pt/C.49 Furthermore, the form of the backward peaks on Pt@Heli-CeO2/C, Pt@Octa-CeO2/C and Pt/C are quite different. A relatively sharp peak is observed for the Pt@Heli-CeO2/C anode, corresponding to the reoxidation of methanol or the removal of adsorbed carbonaceous species.29 The chronoamperometric i–t curves from a N2 saturated 0.5 M H2SO4 and 2.0 M CH3OH aqueous solution at 0.5 V are presented in Fig. 7b. For Pt@Heli-CeO2/C, the current density at 3600 s is 0.089 A mg−1 Pt, which is approximately 8.2 times higher than that of Pt/C (0.011 A mg−1 Pt), and 3.3 times higher than that of Pt@Octa-CeO2/C (0.027 A mg−1 Pt). This obviously enhanced oxidation activity for the Pt@Heli-CeO2/C compared with Pt@Octa-CeO2/C and Pt/C is definitely attributed to the enhancement in the conductivity owing to the interfacial interaction between the helical structured Heli-CeO2 and Pt, which is attributed to the helical structured Heli-CeO2 affording more active oxygen and exposing more active surface sites which is beneficial for the deposition of Pt nanoparticles. This in turn, increases the close contact and synergistic interactions between Pt and the helical structured Heli-CeO2, reducing the aggregation of Pt, as well as increasing the Pt(0) composition in Pt@Heli-CeO2/C.
Conclusions
In summary, the results of the current study demonstrate a facile procedure to transform nano-octahedron CeO2 into helical nanospindle Heli-CeO2. The Heli-CeO2 and Octa-CeO2 promoted Pt/C samples were synthesized successfully by the microwave-assisted polyol synthesis method. The helical CeO2 modified electrode exhibits a higher activity than the octahedron ceria supported electrode and Pt/C. The experimental results show that for methanol electro-oxidation Pt@Heli-CeO2/C possesses 8.2 and 3.2 times higher the activity than Pt/C and Pt@Octa-CeO2/C, respectively. The significant enhancement for activity can be attributed to the following reasons: (1) the unique helical structures are more conductive with better electron transfer than the corresponding octahedral structures due to the intrinsic electronic structure; (2) there are more oxygen-containing species of the helical structured Heli-CeO2 support for Pt anchoring, and more active surface sites of the helical structured CeO2 to strengthen the support–metal interactions and promote the homogenous dispersion of Pt crystallites; (3) reduced aggregation of Pt nanoparticles; (4) increased Pt(0) composition in the helical CeO2 supported electrode. The present study provides a new approach to the design of metal oxide cocatalysts for alcohol electrooxidation application.Acknowledgements
The work was supported by the National Natural Science Foundation of China (Grant no. 51271012)Notes and references
- Y. Y. Chu, Z. B. Wang, Z. Z. Jiang, D. M. Gu and G. P. Yin, Adv. Mater., 2011, 23, 3100 CrossRef CAS PubMed
.
- D. R. Ou, T. Mori, H. Togasaki, M. Takahashi, F. Ye and J. Drennan, Langmuir, 2011, 27, 3859 CrossRef CAS PubMed
.
- J. Zheng, D. A. Cullen, R. V. Forest, J. A. Wittkopft, Z. Zhuang, W. Sheng, J. G. Chen and Y. Yan, ACS Catal., 2015, 5, 1468 CrossRef CAS
.
- L. Zhang and Y. Shen, ChemElectroChem, 2015, 2, 887 CrossRef CAS PubMed
.
- D. H. Kwak, Y. W. Lee, S. B. Han, E. T. Hwang, H. C. Park, M. C. Kim and K. W. Park, J. Power Sources, 2015, 275, 557 CrossRef CAS PubMed
.
- S. C. Zignani, V. Baglio, E. R. Gonzalez and A. S. Arico, ChemElectroChem, 2014, 1, 1403 CrossRef CAS PubMed
.
- Y. Wang, G. Wang, G. Li, B. Huang, J. Pan, Q. Liu, J. Han, L. Xiao, J. Lu and L. Zhuang, Energy Environ. Sci., 2015, 8, 177 CAS
.
- N. Kakati, J. Maiti, S. H. Lee, S. H. Jee, B. Viswanathan and Y. S. Yoon, Chem. Rev., 2014, 114, 12397 CrossRef CAS PubMed
.
- A. Ciftci, S. Eren, D. A. J. M. Ligthart and E. J. M. Hensen, ChemCatChem, 2014, 6, 1260 CAS
.
- R. Nagao, R. G. Freitas, C. D. Silva, H. Varela and E. C. Pereira, ACS Catal., 2015, 5, 1045 CrossRef CAS
.
- D. Xu, S. Bliznakov, Z. Liu, J. Fang and N. Dimitrov, Angew. Chem., Int. Ed., 2010, 49, 1282 CrossRef CAS PubMed
.
- S. J. Liao, K. A. Holmes, H. Tsaprailis and V. I. Birss, J. Am. Chem. Soc., 2006, 128, 3504 CrossRef CAS PubMed
.
- Y. Shen, Z. Zhang, K. Xiao and J. Xi, ChemCatChem, 2014, 6, 3254 CrossRef CAS PubMed
.
- H. Huang, Y. Liu, Q. Gao, W. Ruan, X. Lin and X. Li, ACS Appl. Mater. Interfaces, 2014, 6, 10258 CAS
.
- B. Ruiz-Camacho, H. H. Rodriguez Santoyo, J. M. Medina-Flores and O. Alvarez-Martinez, Electrochim. Acta, 2014, 120, 344 CrossRef CAS PubMed
.
- F. Shi, L. R. Baker, A. Hervier, G. A. Somorjai and K. Komvopoulos, Nano Lett., 2013, 13, 4469 CrossRef CAS PubMed
.
- A. A. Melvin, V. S. Joshi, D. C. Poudyal, D. Khushaani and S. K. Haram, ACS Appl. Mater. Interfaces, 2015, 7, 6590 CAS
.
- W. Zhang, P. Sherrell, A. I. Minett, J. M. Razal and J. Chen, Energy Environ. Sci., 2010, 3, 1286 CAS
.
- V. T. T. Ho, C. J. Pan, J. Rick, W. N. Su and B. J. Hwang, J. Am. Chem. Soc., 2011, 133, 11716 CrossRef CAS PubMed
.
- E. You, R. Guzmán-Blas, E. Nicolau, M. Aulice Scibioh, C. F. Karanikas, J. J. Watkins and C. R. Cabrera, Electrochim. Acta, 2012, 75, 191 CrossRef CAS PubMed
.
- S. Huang, C. Chang and C. Yeh, J. Catal., 2006, 241, 400–406 CrossRef CAS PubMed
.
- M. Takahashi, T. Mori, F. Ye, A. Vinu, H. Kobayashi and J. Drennan, J. Am. Ceram. Soc., 2007, 90, 1291 CrossRef CAS PubMed
.
- Q. Tan, C. Du, Y. Sun, G. Yin and Y. Gao, J. Mater. Chem. A, 2014, 2, 1429 CAS
.
- Y. Y. Chu, J. Cao, Z. Dai and X. Y. Tan, J. Mater. Chem. A, 2014, 2, 4038 CAS
.
- B. Xu, Q. Zhang, S. Yuan, M. Zhang and T. Ohno, Appl. Catal., B, 2015, 164, 120 CrossRef CAS PubMed
.
- X. Yu, L. Kuai and B. Geng, Nanoscale, 2012, 4, 5738 RSC
.
- I. V. Zagaynov, A. V. Vorobiev and S. V. Kutsev, Mater. Lett., 2015, 139, 237 CrossRef CAS PubMed
.
- G. Shen, B. Liang, X. Wang, P. C. Chen and C. Zhou, ACS Nano, 2011, 5, 2155 CrossRef CAS PubMed
.
- S. K. Meher and G. R. Rao, ACS Catal., 2012, 2, 2795 CrossRef CAS
.
- X. Peng, K. Koczkur, S. Nigro and A. Chen, Chem. Commun., 2004, 2872 RSC
.
- A. Pozio, M. De Francesco, A. Cemmi, F. Cardellini and L. Giorgi, J. Power Sources, 2002, 105, 13 CrossRef CAS
.
- X. L. Sui, Z. B. Wang, C. Z. Li, J. J. Zhang, L. Zhao and D. M. Gu, J. Power Sources, 2014, 272, 196 CrossRef CAS PubMed
.
- M. Lo Faro, R. M. Reis, G. G. A. Saglietti, A. G. Sato, E. A. Ticianelli, S. C. Zignani and A. S. Aricò, ChemElectroChem, 2014, 1, 1395 CrossRef CAS PubMed
.
- X. L. Sui, Z. B. Wang, C. Z. Li, J. J. Zhang, L. Zhao, D. M. Gu and S. Gu, J. Mater. Chem. A, 2015, 3, 840 CAS
.
- P. Tarakeshwar, J. L. Palma, G. P. Holland, P. Fromme, J. L. Yarger and V. Mujica, J. Phys. Chem. Lett., 2014, 5, 3555 CrossRef CAS
.
- Y. Zheng, L. Lin, X. Ye, F. Guo and X. Wang, Angew. Chem., Int. Ed., 2014, 53, 11926 CrossRef CAS PubMed
.
- S. Sun, G. Zhang, D. Geng, Y. Chen, R. Li, M. Cai and X. Sun, Angew. Chem., Int. Ed., 2011, 50, 422 CrossRef CAS PubMed
.
- E. Lee and A. Manthiram, J. Phys. Chem. C, 2010, 114, 21833 CAS
.
- J. Carrasco, D. López-Durán, Z. Liu, T. Duchoň, J. Evans, S. D. Senanayake, E. J. Crumlin, V. Matolín, J. A. Rodríguez and M. V. Ganduglia-Pirovano, Angew. Chem., Int. Ed., 2015, 127, 1 CrossRef PubMed
.
- L. X. Ding, C. L. Liang, H. Xu, A. L. Wang, Y. X. Tong and G. R. Li, Adv. Mater. Interfaces, 2014, 1, 1400005 Search PubMed
.
- X. Wang, D. Liu, S. Song and H. Zhang, J. Am. Chem. Soc., 2013, 135, 15864 CrossRef CAS PubMed
.
- Z. Z. Jiang, Z. B. Wang, W. L. Qu, D. M. Gu and G. P. Yin, Appl. Catal., B, 2012, 123–124, 214 CrossRef CAS PubMed
.
- J. A. Haber, E. Anzenburg, J. Yano, C. Kisielowski and J. M. Gregoire, Adv. Energy Mater., 2015, 5, 1402307 Search PubMed
.
- Y. Zhou, N. J. Lawrence, T. S. Wu, J. Liu, P. Kent, Y.-L. Soo and C. L. Cheung, ChemCatChem, 2014, 6, 2937 CrossRef CAS PubMed
.
- F. ç. Larachi, J. Pierre, A. Adnot and A. Bernis, Appl. Surf. Sci., 2002, 195, 236 CrossRef CAS
.
- A. S. Aricò, A. K. Shukla, H. Kim, S. Park, M. Min and V. Antonucci, Appl. Surf. Sci., 2001, 172, 33 CrossRef
.
- A. X. Yin, X. Q. Min, W. Zhu, H. S. Wu, Y. W. Zhang and C. H. Yan, Chem. Commun., 2012, 48, 543 RSC
.
- X. Wang, X. Li, D. Liu, S. Song and H. Zhang, Chem. Commun., 2012, 48, 2885 RSC
.
- A. M. Hofstead-Duffy, D.-J. Chen, S. G. Sun and Y. J. Tong, J. Mater. Chem., 2012, 22, 5205 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: TG result of CeO2-Heli-P; H2-TPR and N2 adsorption–desorption isotherm results of the obtained ceria. See DOI: 10.1039/c5ra09047d |
| This journal is © The Royal Society of Chemistry 2015 |