Copper-catalyzed cross-coupling reactions for C–P bond formation
Abstract
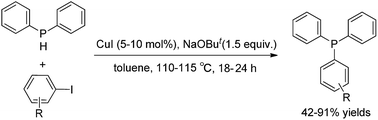
Phosphorus compounds are important compounds in many areas. This review briefly discusses copper-catalyzed C–P bond formation reactions. In the presence of a copper catalyst, various C–P bonds can be formed. C–P bond formation reactions are classified based on the different hybridization of the carbon which is connected with the P atom. In addition, the challenges and opportunities of these reactions are also discussed.

 Please wait while we load your content...
Please wait while we load your content...