Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermally flexible epoxy/cellulose blends mediated by an ionic liquid†
N.
Hameed
*ab,
J.
Bavishi
ab,
J.
Parameswaranpillai
c,
N. V.
Salim
ab,
J.
Joseph
b,
G.
Madras
d and
B. L.
Fox
ab
aCarbon Nexus, Deakin University, Geelong, Victoria 3216, Australia
bInstitute for Frontier Materials, Deakin University, Geelong, Victoria 3216, Australia. E-mail: nishar.hameed@deakin.edu.au
cDepartment of Polymer Science and Rubber Technology, Cochin University of Science and Technology, Cochin, Kerala 682 022, India
dDepartment of Chemical Engineering, Indian Institute of Science, Bangalore 560012, India
First published on 8th June 2015
Abstract
Blends between the widely used thermoset resin, epoxy, and the most abundant organic material, natural cellulose are demonstrated for the first time. The blending modification induced by charge transfer complexes using a room temperature ionic liquid, leads to the formation of thermally flexible thermoset materials. The blend materials containing low concentrations of cellulose were optically transparent which indicates the miscibility at these compositions. We observed the existence of intermolecular hydrogen bonding between epoxy and cellulose in the presence of the ionic liquid, leading to partial miscibility between these two polymers. The addition of cellulose improves the tensile mechanical properties of epoxy. This study reveals the use of ionic liquids as a compatible processing medium to prepare epoxy thermosets modified with natural polymers.
Cellulose is the most abundant organic material and important renewable resource on the Earth.1 Cellulose is biodegradable and readily available for the manufacturing of sustainable materials at low cost and energy consumption.1 Commercially, cellulose is one of the widely used polymers with applications ranging from packaging to biomaterials and high value electrode devices.2 Structurally, cellulose is a very stable compound insoluble in water or most organic solvents due to the large network of intermolecular hydrogen bonding.3 There are only a few solvent systems available for the direct dissolution of cellulose such as N,N-dimethylacetamide/lithium chloride tetra butyl ammonium fluoride/dimethyl sulfoxide, N-methylmorpholine-N-oxide monohydrate, etc., however most of these solvents are not very environment friendly.4,5 Moreover cellulose and its derivatives have been also blended with synthetic polymers such as polyethylene oxide,6 polyvinyl chloride,7 nylon8etc. using solvents other than ionic liquids. Nevertheless blends of cellulose with a thermosetting polymer such as epoxy have never been reported.
Epoxy resins, on the other hand are a class of important thermosetting polymers widely used in coatings, adhesives, paints, and as matrix material for automotive and aerospace composites. Cured epoxy resins are highly brittle and have low impact resistance due to their highly cross-linked structure.9 The properties of epoxy can be tuned by blending with other polymers and the blend morphology plays a significant role in the final properties of such thermosets.10–13 A large number of epoxy blends have been studied in the past by incorporating secondary rubbery segment or thermoplastic that phase separates from the matrix during curing, leading to different morphologies with encouraging properties.14–16 Epoxy/cellulose fiber composites and epoxy blended with cellulose derivatives such as cellulose acetate and hydroxyethyl cellulose acetate have been extensively investigated.17–20 However, epoxy and natural cellulose blends have never been investigated even though they are the most commonly used polymeric materials. This is primarily due to the fact that cellulose is insoluble in epoxy resin and there is no suitable solvent to dissolve cellulose in epoxy resin. The insolubility between cellulose and epoxy can be attributed to the fact that they both are structurally stabilized by forming inter-associated hydrogen bonds. To our knowledge, there has been no solvent or process to break the intermolecular hydrogen bonding within cellulose or epoxy and form intermolecular interactions between them. For this reason, research on these materials has been limited to composites and nanocomposites where various forms of cellulose were used as an insoluble filler reinforcement material in epoxy.21
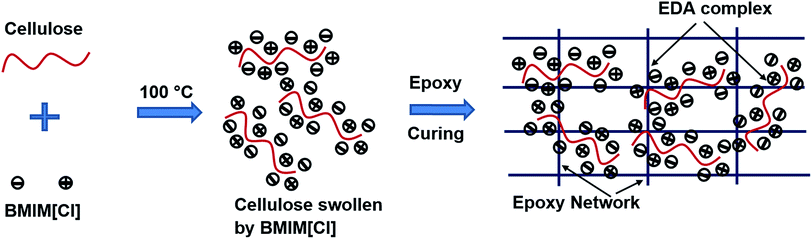
Here, for the first time, we report the dissolution and blending of epoxy and cellulose using room temperature ionic liquid (IL) as a co-additive solvent. In the last decade, IL was identified as a new class of solvent both for the regeneration and chemical modification of cellulose. In the past, we have extensively used ILs for the blending modification of natural polymer blends.5,24 Among, imidazolium based ILs, 1-butyl-3-methylimidazolium chloride BMIM[Cl] is reported to be the most efficient and widely used IL in terms of dissolving cellulose.25 Though BMIM[Cl] is considered as a toxic chemical, no adverse effect of using these chemical in these blends as it becomes a part of the epoxy cross-linked network. In the present study, diglycidyl ether of bisphenol A (DGEBA) epoxy blends containing cellulose up to 40 wt% were prepared from 1-butyl-3-methylimidazolium chloride BMIM[Cl]. In this work, we investigate how the intractable natural polymer cellulose can be blended with epoxy using the IL solvent BMIM[Cl]. Epoxy and cellulose were blended together in BMIM[Cl] to study the miscibility and hydrogen bonding interactions between these two polymers and also to examine the phase separated morphologies in these blends.
The interactions among epoxy/IL and epoxy/cellulose groups were analysed using IR spectroscopy. We assume that a charge transfer complexation occurred between ions of IL and hydroxyl groups of cellulose and epoxy network. We created classic epoxy networks of DGEBA cured by a stoichiometric mixture of diamine curing agent, 4,4′-methylenedianiline (MDA) and used charge transfer complexation reaction to confine the bulky ILs within the permanently cross-linked networks. The EDA complexation of ILs has been reported with intractable natural polymers such as cellulose, during their dissolution process.26,27 In epoxy and cellulose, the hydroxyl oxygen atom acts as the donor and the hydrogen atom as the acceptor whereas in the IL, the charged species BMIM+ acts as the acceptor and Cl− as the donor (Fig. 1). The two centers of the species must be positioned close enough to allow the charge transfer and complexation. The complexation leads to the uniform confinement of ILs molecules within the epoxy/cellulose chains and thus all components become part of the cross-linked network. Or in other words IL link epoxy and cellulose chains by forming EDA complexes with both polymers.
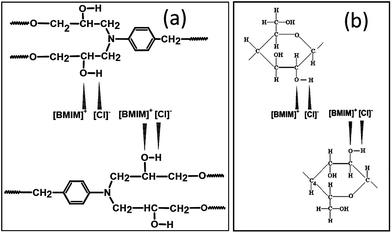 | ||
| Fig. 1 The proposed formation of EDA complexes between (a) epoxy/BMIM[Cl]22 and (b) cellulose/BMIM[Cl]23 (the chemical structure of the BMIM[Cl] is given in Scheme S1†). | ||
The EDA complex formation results in the stretching of hydroxyl groups of epoxy network which was monitored by infrared spectroscopy (Fig. S1a†). The presence of hydroxyl groups makes epoxy a self-associating polymer. The hydroxyl stretching region of spectra shows a broad band (3390 cm−1) and a shoulder band (3565 cm−1) corresponding to the stretching vibrations of self-associated hydroxyl groups and non-associated free hydroxyl groups, respectively.28 However, upon charge transfer complexation in the presence of ILs, the peak corresponding to the free hydroxyl groups disappears and associated hydroxyl stretching band at 3390 cm−1 is shifted to lower frequencies indicating hydroxyl groups of epoxy are stretched due to complexation. This redshift also confirms that the bonding in EDA complexes is stronger than the self-associated hydrogen bonding in cured epoxy network, providing materials with modified and more flexible network structure.
In order to dissolve cellulose, the inter and intra-molecular interactions have to be disrupted. It is reported that high chloride concentration in BMIM[Cl] is efficient in breaking down the highly networked hydrogen bonding and thereby dissolving cellulose by forming complexes.25 The inter and intra-molecular hydrogen bonding interactions in epoxy/cellulose blends were determined by FTIR spectroscopy (Fig. S1b†). The broad band in the 3000–3500 cm−1 region can be assigned to a wide distribution of the stretching vibration of free, self-associated and inter-associated hydroxyl groups epoxy and cellulose. When blending with cellulose, the peak (3315 cm−1) corresponding to the complexed epoxy/IL network shifted towards higher wavenumber region (3360 cm−1). This upward shifting indicates the existence of hydrogen bonding and/or EDA complexation between epoxy/cellulose hydroxyl groups and IL. Also small amounts of peak broadening was observed in blends containing higher amounts of cellulose (especially at 20 and 30 wt%) which also indicates the presence of hydrogen bonding interactions in these blends. The interaction between hydroxyl group of epoxy and hydroxyl group of cellulose mediated via IL is assumed to be the driving force of the partial miscibility of between these polymers.
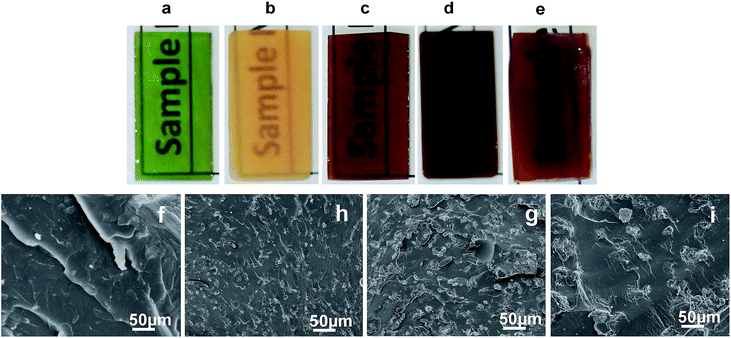
The blends were all transparent at 100 °C before curing, thus indicating the macroscopic homogeneity of the mixtures in the molten state. The MDA-cured ER/cellulose networks were also completely or near-transparent with cellulose content up to 10 wt%; however, they appeared translucent or opaque with higher cellulose content at room temperature as shown in Moreover, the specimens were all appeared in different colors and was very obvious when compared with neat resin (green) and cellulose added blends (brown shades). This is assumed to be raised from the charge transfer reactions in the blends.29
Fig. 2a–e. The morphologies of the cured ER/cellulose blends were observed using SEM and the cryo-fractured images are also shown in Fig. 2f–i. The microstructure of neat epoxy sample shows smooth surface with distinct line of fracture propagation (image not shown). This is due to inherent brittleness of epoxy resin. The blends with 10 wt% cellulose show a homogenous morphology with no apparent microphase of cellulose (Fig. 2f) indicating complete miscibility between epoxy and cellulose. The blends containing 20 wt% and above cellulose content show micro phase separated structures. This indicates the absence of homogeneous miscibility between epoxy and cellulose. The SEM images of 20 wt% sample show cellulose microphase of ∼10 μm are embedded within epoxy matrix and the size of the cellulose phase increases with increase in cellulose concentration in the network (Fig. 2g–i).
The ER/cellulose blends containing IL possess interesting phase behavior since IL acts like intermediating link between the two dissolved phases in the network structure. In the DSC thermograms (Fig. S2†), the neat epoxy containing 40 wt% IL shows a glass transition temperature (Tg) at 70 °C and Tg of cellulose phase could not be examined from the DSC experiments. However, the Tg of cellulose in dielectric, NMR and other studies is described to be in the high temperature region (∼250 °C).30 It can be seen that in the blends, the Tg of epoxy is shifted to lower temperatures with addition of the cellulose. For example, the Tg of epoxy in the blends containing 40 wt% cellulose is 55 °C.
In binary blends, a decrease of the Tg is usually interpreted as increase of the mobility of the chain segments of epoxy network, indicating increase in free volume of system.31 Reduction of Tg with fillers addition is usually observed when very small size fillers are used in nanocomposites.32,33 One possibility for the reduction in Tg could be the preferential interaction between the cellulose, IL and cross-linked network that would lead to non-stoichiometric balance between epoxy monomer and curing agent, thus leading the partial inhibition of crosslinking reaction.
The phase behavior of the blends was also investigated using DMTA. The storage modulus (G′) and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ versus temperature curves of the neat epoxy resin and blends with cellulose up to 40 wt% tested at 1 Hz is shown in Fig. S3.† All the samples show well defined relaxation peak corresponding to their Tg. The Tg values of the blends decrease with increase in cellulose content. Young's modulus of ER is slightly increased by of ∼8–10% by the addition of 20 wt% cellulose but a reduction in modulus was observed in 30 wt% and above cellulose content.
δ versus temperature curves of the neat epoxy resin and blends with cellulose up to 40 wt% tested at 1 Hz is shown in Fig. S3.† All the samples show well defined relaxation peak corresponding to their Tg. The Tg values of the blends decrease with increase in cellulose content. Young's modulus of ER is slightly increased by of ∼8–10% by the addition of 20 wt% cellulose but a reduction in modulus was observed in 30 wt% and above cellulose content.
Tensile strength and elongation at break are important mechanical properties of the ER/cellulose blends, which mainly depend on blend components and interfacial interaction between them. Table 1 summarizes the values tensile properties of ER/cellulose blends. Addition of cellulose considerably improves the tensile strength of epoxy. The blends containing 10 wt% of cellulose show the highest tensile strength of 66 MPa, which is 37% higher than neat epoxy containing 40 wt% IL. The incorporation of a small amount of the cellulose can effectively enhance the tensile strength of epoxy thermosets. The increase in tensile strength can be attributed to the miscibility between epoxy and 10 wt% of cellulose. The tensile strength decreases in blends above 10 wt% of cellulose owing to partial miscibility or microphase separation at these blend compositions. Moreover, the percentage elongation of the blends was decreased by the increase in cellulose content. The thermal decomposition behavior of ER/cellulose blends containing IL was also examined (Fig. S4†). The onset decomposition temperature (Td) of neat epoxy containing IL is 286 °C. It can be observed that the Td of ER/cellulose blends decreases marginally with increase in cellulose content. The least thermal stability was exhibited by the blend containing 40 wt% cellulose at 260 °C.
| Cellulose (wt%) | Tensile strength (MPa) | % elongation |
|---|---|---|
| 0 | 47.15 ± 4 | 3.5 ± 0.5 |
| 10 | 66.01 ± 8 | 3.2 ± 0.29 |
| 20 | 54.68 ± 5 | 2.1 ± 0.28 |
| 30 | 39.42 ± 1 | 1.2 ± 0.3 |
| 40 | 19.87 ± 8 | 0.5 ± 0.11 |
The neat epoxy and epoxy/cellulose blends, all containing 40 wt% of IL were thermally flexible. The materials can be reversibly bent or twist many times by applying local heat and force. Fig. 3 shows the fusilli and curved shape produced by heating blends containing 10 wt% cellulose. No significant difference in flexibility was observed with cellulose content. We assume that the thermal flexibility of epoxy/cellulose blends arise from the confinement of IL molecules in between the highly cross-linked network. As previously explained the strong interaction between bulky IL ions and the epoxy/cellulose hydroxyl groups links the brittle chains network. When heated, these bonds break and the presence of detached bulky ions leads to flexible networks.
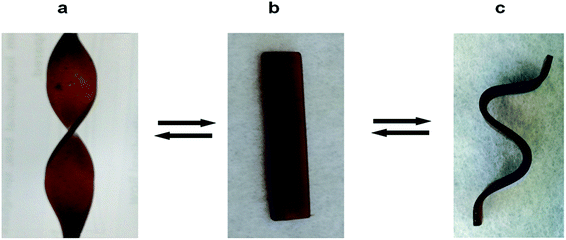 | ||
| Fig. 3 The reversible thermal flexibility of the epoxy/cellulose blends materials shown by making fusilli (a) and curved shape (c) from a rectangular piece of epoxy/cellulose (10 wt%) blends. | ||
Epoxies are inherently brittle and making flexible and formable epoxy networks is one of the stimulating research topics these days. Recent reports regarding the formation of recyclable and thermally malleable epoxy materials have been welcomed both by the scientific and industrial community. The present work reports a method to make flexible epoxy thermosets by introducing ionic liquid which acts as a link between the cross-linked networks by forming EDA complexes. Natural cellulose was added as a renewable and inexpensive polymer to improve the mechanical properties of epoxy/IL network. Here the addition of 10 wt% of cellulose lead to 37% increase in the tensile strength with no significant reduction in elongation.
The mechanism of microphase separation in epoxy/cellulose blends in the presence of IL is given in Fig. 4. It is assumed that the mixing of various amounts of cellulose in IL at 100 °C leads to miscible or swollen cellulose solution. Adding epoxy and further heating and cross linking reaction results in the formation of EDA complexes between IL ions and epoxy hydroxyl groups as well as the phase separation of cellulose occurs within the system during curing reaction. The size of cellulose phase depends on the concentration of the cellulose component in the system.
Conclusions
Partially miscible epoxy blends modified with natural cellulose were prepared for the first time. The miscibility between epoxy and cellulose was induced by the introduction of BMIM[Cl] ionic liquid which acts as a coupling agent by forming EDA complexes between the two polymers. The blending modification leads to transparent, thermally flexible thermosets with improved tensile strength. This study shows a processing route by which miscible or partially miscible epoxy blends can be prepared with other natural polymers such as wool, silk, etc.Author contributions
All authors contributed to the scientific discussions regarding the research. J.B. performed sample preparation, DSC, FTIR and DMTA measurements. N.V.S. and N.H. discussed and developed dissolution mechanism. J.J. performed the SEM experiments and discussions. G.M., J.P. and B.L.F. contributed to the results and discussions including writing and editing of the manuscript. N.H. conceived the idea, coordinated the project and wrote the manuscript.Conflict of interest
The authors declare no competing financial interests.Acknowledgements
N.H. acknowledges the Australian Academy of Science for the AIECR funding and VESKI for the Victoria Fellowship. J.P. acknowledges the Department of Science and Technology, Government of India, for financial support under an INSPIRE Faculty Fellowship (IFA-CH-16). The present work was carried out with the support of the Deakin Advanced Characterisation Facility.References
- K. Kamide, Cellulose and Cellulose Derivatives, Elsevier, The Netherlands, 2005 Search PubMed.
- Biopolymers: utilizing nature's advanced materials, ed. S. H. Imam, R. V. Greene and B. R. Zaidi, ACS symposium series, no. 723, American Chemical Society, Washington, DC, 1999 Search PubMed.
- V. L. Finkenstadt and R. P. Millane, Macromolecules, 1998, 31, 7776 CrossRef CAS.
- S. Sen, J. D. Martin and D. S. Argyropoulos, ACS Sustainable Chem. Eng.,
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2013, 1, 858 Search PubMed.
2013, 1, 858 Search PubMed. - N. Hameed and Q. Guo, Cellulose, 2010, 17, 803 CrossRef CAS.
- Y. Nishio, N. Hirose and T. Takahashi, Polym. J., 1989, 21, 347 CrossRef CAS.
- Y. Nishio and R. S. J. Manley, Macromolecules, 1988, 21, 1270 CrossRef.
- Y. Nishio and R. S. J. Manley, Polym. Eng. Sci., 1990, 30, 71 CAS.
- C. A. May and G. Y. Tanaka, Epoxy resin chemistry and technology, New York, Marcel Dekker, 1973 Search PubMed.
- S. Kar and A. K. Banthia, J. Appl. Polym. Sci., 2005, 96, 2446 CrossRef CAS.
- B. Francis, V. L. Rao, G. V. Poel, F. Posada, G. Groeninckx and R. Ramaswamy, Polymer, 2006, 47, 5411 CrossRef CAS.
- K. Mimura, H. Ito and H. Fujioka, Polymer, 2000, 41, 4451 CrossRef CAS.
- N. Hameed, P. A. Sreekumar, B. Francis, W. Yang and S. Thomas, Composites, Part A, 2007, 38, 2422 CrossRef.
- M. L. Arias, P. M. Frontini and R. J. J. Williams, Polymer, 2003, 44, 1537 CrossRef CAS.
- N. Salmon, V. Carlier, J. Schut, P. M. Remiro and I. Mondragon, Polym. Int., 2005, 54, 667 CrossRef CAS.
- B. Francis, R. Ramaswamy, V. L. Rao, S. Jose, S. Thomas and K. V. S. N. Raju, Polym. Eng. Sci., 2005, 45, 1645 CAS.
- A. K. Bledzki and J. Gassan, Prog. Polym. Sci., 1999, 24, 221 CrossRef CAS.
- R. Mahendrana, R. Malaisamya and D. Mohan, J. Macromol. Sci., Part A: Pure Appl.Chem., 2002, 39, 1025 CrossRef.
- Q. Dai, J. Chen and Y. Huang, J. Appl. Polym. Sci., 1998, 70, 1159 CrossRef CAS.
- R. Mahendrana, R. Malaisamya and D. Mohan, Eur. Polym. J., 2004, 40, 623 CrossRef.
- H. Almri and I. M. Low, Polym. Test., 2012, 31, 620 CrossRef.
- N. Hameed, N. V. Salim, T. R. Walsh, J. S. Wiggins, P. M. Ajayan and B. L. Fox, Chem. Commun., 2015, 51, 9903 RSC.
- S. Xu and J. C. Meredith, Polymer, 2013, 54, 6589 CrossRef CAS.
- N. Hameed and Q. Guo, Carbohydr. Polym., 2009, 78(4), 999–1004 CrossRef CAS.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974 CrossRef CAS PubMed.
- A. Pinkert, K. N. Marsh, S. Pang and M. P. Staiger, Chem. Rev., 2009, 109, 6712 CrossRef CAS PubMed.
- L. Feng and Z.-I. Chen, J. Mol. Liq., 2008, 142, 1 CrossRef.
- N. Hameed, Q. Guo, Z. Xu, T. L. Hanley and Y. W. Mai, Soft Matter, 2010, 6, 6119 RSC.
- P. M. S. Monk, N. M. Hodgkinson and R. D. Partridge, Dyes Pigm., 1999, 43, 241 CrossRef CAS.
- J. Kubat and C. Pattyrante, Nature, 1967, 215, 390 CrossRef CAS.
- Y. Sun, Z. Zhang, K.-S. Moon and C. P. Wong, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 3849 CrossRef CAS.
- S. G. Prolongo, M. Campo, M. R. Gude, R. Chaos-Morán and A. Ureña, Compos. Sci. Technol., 2009, 69, 349 CrossRef CAS.
- L. Sun, G. L. Warren, J. Y. O. OReilly, W. N. Everett, S. M. Lee and D. Davis, Carbon, 2008, 46, 320 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation of materials, FTIR, DSC, DMTA and TGA results. See DOI: 10.1039/c5ra05900c |
| This journal is © The Royal Society of Chemistry 2015 |