Morphological study of microwave-assisted facile synthesis of gold nanoflowers/nanoparticles in aqueous medium and their catalytic application for reduction of p-nitrophenol to p-aminophenol
Abstract
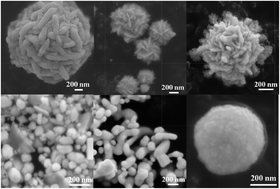
The present work reports a facile, microwave assisted one-pot synthesis of gold (Au) nanostructures with different morphologies. The Au nanoparticles were synthesized using an aqueous solution of AuCl3 and dimethyl sulfoxide (DMSO) as a structure directing agent. DMSO plays a crucial role in the synthesis as it acts as a reducing agent and capping agent. It also controls the crystal morphology of Au nanoparticles. The phase identification, morphology and elemental composition of the synthesized Au nanoparticles were studied using XRD, FEG-SEM, HRTEM and EDS respectively. In addition, we showed the catalytic activity of Au nanoparticles for the reduction of p-nitrophenol to p-aminophenol in the presence of sodium borohydride at room temperature. The catalytic activity was evaluated using UV-Vis spectroscopy. This is a simple, rapid, additive free, economic and greener approach for the synthesis of Au nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...