Nanocomposite Li3V2(PO4)3/carbon as a cathode material with high rate performance and long-term cycling stability in lithium-ion batteries
Peixun Xiongab,
Lingxing Zengab,
Huan Liab,
Cheng Zhengab and
Mingdeng Wei*ab
aState Key Laboratory of Photocatalysis on Energy and Environment, Fuzhou University, Fuzhou, Fujian 350002, China. E-mail: wei-mingdeng@fzu.edu.cn; Tel: +86-591-83753180
bInstitute of Advanced Energy Materials, Fuzhou University, Fuzhou, Fujian 350002, China
First published on 24th June 2015
Abstract
In the present work, nanocomposite Li3V2(PO4)3/carbon is successfully synthesized by combining a sol–gel method and a nanocasting route, and then it is characterized by means of X-ray diffraction (XRD), thermogravimetric analysis (TG), N2 adsorption–desorption, and transmission electron microscopy (TEM). Furthermore, this nanocomposite is used as a cathode material for Li-ion intercalation and exhibits large reversible capacity, high rate performance and excellent long-term cycling stability. For instance, a large reversible capacity of 95 mA h g−1 and an average Coulombic efficiency of 99.1% can be maintained even after 3000 cycles at a high rate of 20C in the potential range of 3.0–4.3 V. Moreover, the Li3V2(PO4)3/C nanocomposite delivered a large capacity of 127 mA h g−1 at a high rate of 10C in the voltage range of 3.0–4.8 V. The super results might be attributed to the unique hierarchical architecture of the Li3V2(PO4)3/carbon nanocomposite.
Introduction
With increasing concerns regarding environmental protection and global warming, there is a strong and increasing demand for the development of power sources for hybrid electric vehicles (HEVs) and renewable energy systems.1 Lithium-ion batteries (LIBs) have widely been applied as clean and renewable power sources for portable electronic devices such as digital videos, notebook PCs, and have also been proposed for use in electric vehicles and large-scale energy storage.2–5 However, the performance of current LIBs cannot meet the requirements of electric vehicles in terms of high rate performance and long term cycling stability.6–13Recently, Li3V2(PO4)3 has been identified as a promising candidate cathode material for LIBs due to its good ion mobility, high theoretical capacity and high operating voltage.14–21 Despite these advantages, Li3V2(PO4)3 still suffers from the problem of poor capacity and cycling stability at high rate because of its low electronic conductivity as well as the side reaction between the active material and organic electrolyte. It is well known that small particle size, carbon coating, and doping of other metal ions are beneficial for improving the electrochemical properties of Li3V2(PO4)3.22 Among various approaches, carbon coating plays an important role due to the advantages arising from the unique properties of carbon, such as unique physical properties, chemical and electrochemical stability.23–27 Moreover, nano-size provides a shorter path for Li-ion and electron transport, which facilitates improved kinetics.28–33 However, the preparation of a Li3V2(PO4)3/carbon composite (Li3V2(PO4)3/C) with nano-size is not an easy issue. The formation of a Li3V2(PO4)3 phase with an electrical conductive carbon layer generally involves high sintering temperature and long sintering time, in which Li3V2(PO4)3 tends to grow and aggregate into large grains.
In recent studies, mesoporous carbon (MC) has been used as a promising nanoreactor for fabricating nanomaterials with high Li-ion storage capability and stability.34–39 The mesochannel and large surface area of MC shortens the distance of Li-ion diffusion and its high conductivity is in favor to electron transmission. On the other hand, it has large pore volumes, which offer a better accommodation of the strain and volume changes during the charge–discharge process. These results encouraged us to extend our studies to the investigation of nanocomposite composed of mesoporous carbon and Li3V2(PO4)3. In the present work, the nanocomposite Li3V2(PO4)3/C was synthesized by combining sol–gel method and nanocasting route, and exhibited excellent rate performance and long-term cycling stability for Li-ion intercalation. Furthermore, the relationships between the intrinsic of nanocomposite Li3V2(PO4)3/C and the electrochemical properties were also investigated in detail.
Experimental
1. Preparation and characterizations
The synthesis process of mesoporous carbon is similar to that described by Ryoo.40,41 For a typical synthesis of Li3V2(PO4)3/C nanocomposite, 0.244 g of Li2CO3, 0.759 g of NH4H2PO4, 0.515 g NH4VO3 and 0.1 g citric acid were dispersed in 20 mL of hot distilled water under ultrasonication for 0.5 h to form a bright yellow solution. Then 0.1 g of MC powder was introduced to the solution, under ultrasonication for 30 min. Then the mixture was vigorous stirring for 12 h at 40 °C. After the obtained mixture was heated at 75 °C for 1 h, it was ground and then calcined at 700 °C in Ar for 5 h to obtain the Li3V2(PO4)3/C nanocomposite sample.XRD patterns were recorded on a PANalytical X'Pert spectrometer using the Co Kα radiation (λ = 1.789 Å), and the data would be changed to Cu Kα data. SEM and TEM were taken on a Hitachi 4800 instrument and a FEI F20 S-TWIN instrument, respectively. N2 adsorption–desorption analysis was measured on a Micromeritics ASAP 2020 instrument, pore volumes were determined using the adsorbed volume at a relative pressure of 0.99, multipoint Brunauer–Emmett–Teller (BET) surface area was estimated from the relative pressure range from 0.06 to 0.3. To determine the actual amount of carbon in the nanocomposites, thermogravimetric analysis (TGA) was performed using a CHNS/O analyzer (PE 2400II, Perkin Elmer, America) in air atmosphere.
2. Electrochemical measurements
For the electrochemical measurement, 80 wt% active materials (Li3V2(PO4)3/C nanocomposite) was mixed and grounded with 10 wt% polyvinylidene fluoride (PVDF) powder as a binder and 10 wt% acetylene back carbon (AB) powder as the conductive assistant materials. The mixture was spread and pressed on Al foil circular flakes as the working electrode (WE), and dried at 120 °C for 12 h under the vacuum conditions. Metallic lithium foils were used as the negative electrodes. The electrolyte was 1 M LiPF6 in a 1/1/1 (volume ratio) mixture of ethylene carbonate (EC), ethylene methyl carbonate (EMC) and dimethyl carbonate (DMC). The separator was UP 3093 (Japan) micro-porous polypropylene membrane. The specific capacity values of Li3V2(PO4)3/C nanocomposite are calculated on the basis of the mass of Li3V2(PO4)3. In average, the amount of active material in test cells was ca. 1–2 mg. Without any specific explanation, the galvanostatic charge and discharge experiment was performed in the range of 3.0–4.3 V (1C = 133 mA h g−1) and 3.0–4.8 V (1C = 198 mA h g−1) (vs. Li+/Li) at room temperature, respectively. The cells were assembled in a glove box filled with highly pure argon gas (O2 and H2O levels < 1 ppm), and charge–discharge tests were performed on a Land automatic batteries tester (Land CT 2001A, Wuhan, China).Results and discussion
Fig. 1 shows the XRD pattern of Li3V2(PO4)3/C nanocomposite. As seen from Fig. 1, all diffraction peaks can be ascribed to the characteristic peaks of monoclinic Li3V2(PO4)3 (JCPDS 080-1515), confirming that Li3V2(PO4)3/C nanocomposite can be obtained. The citric acid can promote raw materials to reach atomic level mixing and homogenously trap around the mesoporous carbon channels, which reduces particle size to nanoscale level. Apart from reducing metal oxide during the reaction process, mesoporous carbon can be involved in controlling the particle growth and providing a conductive network to facilitate electron transfer, and as a result, the electrode performances could be enhanced. Based on N2 adsorption–desorption analysis, it was found that the BET surface area and pore volume were 134 m2 g−1 and 0.22 cm3 g−1 for Li3V2(PO4)3/C nanocomposite.TEM images of Li3V2(PO4)3/C nanocomposite are presented in Fig. 2. As shown in Fig. 2a and b, most of Li3V2(PO4)3 nanoparticles were partially loaded inside and outside channels of mesoporous carbon matrix in the Li3V2(PO4)3/C nanocomposite. It can also be found that the size of Li3V2(PO4)3 nanoparticles in the nanocomposite ranged from 30 to 80 nm. Fig. 2c shows the HRTEM images of Li3V2(PO4)3/C nanocomposite. A thin coating layer of carbon was formed on the surface of particles and its thickness was estimated to be ca. 2–3 nm. It also showed that these nanoparticles were high crystalline and the lattice fringe was found to be approximately 0.387 nm, corresponding to the d120-spacing of monoclinic Li3V2(PO4)3. The chemical composition of the Li3V2(PO4)3/C nanocomposite were measured by EDS, as depicted in Fig. 2d. It was also confirmed that the sample existed carbon, oxygen, phosphorus and vanadium elements. The Li element cannot be detected because of the detection limit of EDS. The presence of oxygen mainly came from Li3V2(PO4)3, and a little from atmospheric O2, or CO2 adsorbed on the surface of the sample.
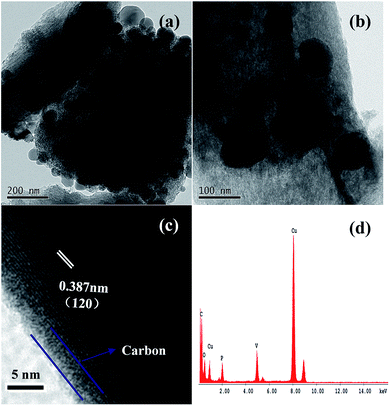 | ||
| Fig. 2 (a and b) TEM images of Li3V2(PO4)3/C nanocomposite; (c) HRTEM images of Li3V2(PO4)3/C nanocomposite; (d) EDS spectra obtained from (b) Li3V2(PO4)3/C nanocomposite. | ||
To confirm the amount of carbon in Li3V2(PO4)3/C nanocomposite, TGA was carried out in air and the result is depicted in Fig. 3. The samples were heated from 50 to 700 °C at a rate of 5 °C min−1. The weight loss below 150 °C was probably due to the evaporation of adsorbed moisture, considering the relatively high surface area of the samples. As can be seen from Fig. 3a, the maximum weight loss of MC samples was taken place at 400–550 °C. According to Fig. 3b, the content of carbon was estimated to be ca. 11.5 wt% for the Li3V2(PO4)3/C nanocomposite.
The electrochemical behavior of Li3V2(PO4)3/C nanocomposite electrode was measured by CV at a scanning rate of 0.5 mV s−1 between 3.0 and 4.3 V. As depicted in Fig. 4a, the peaks of Li3V2(PO4)3/C nanocomposite are sharp and have a high intensity. The well-defined anodic and cathodic peaks were observed at around 3.57/3.59, 3.65/3.67 and 4.04/4.07 V. Monoclinic Li3V2(PO4)3 contains three independent lithium sites. Such three pairs of charge–discharge plateaus were associated with two Li+ insertion/extraction into/out of the monoclinic Li3V2(PO4)3 lattice based on the V3+/V4+ redox couple, respectively. The Li+ insertion/extraction into/out of Li3V2(PO4)3 can be written as the following equations.5,42,43
| Li3V2(PO4)3 ↔ Li2.5V2(PO4)3 + 0.5Li+ + 0.5e− | (1) |
| Li2.5V2(PO4)3 ↔ Li2V2(PO4)3 + 0.5Li+ + 0.5e− | (2) |
| Li2V2(PO4)3 ↔ LiV2(PO4)3 + Li+ + e− | (3) |
The potential difference between the anodic peaks and the corresponding cathodic peaks is small in Li3V2(PO4)3/C nanocomposite, indicating an alleviated polarization and facile extraction/insertion of Li+ in Li3V2(PO4)3/C nanocomposite. Moreover, the second and sixth CV curves remained steady, indicating the highly reversible performance of Li3V2(PO4)3/C nanocomposite electrode.
Fig. 4b shows the cycling performances of Li3V2(PO4)3/C nanocomposite at a current rate of 1C in a potential window of 3.0–4.3 V (1C = 133 mA h g−1). It exhibits an initial discharge capacity of 110 mA h g−1 and the charge capacity of 132 mA h g−1, corresponding to a Coulombic efficiency of 83.3%, which is relatively higher than previous Li3V2(PO4)3-based materials.21 The Coulombic efficiency of the Li3V2(PO4)3/C nanocomposite was up to 98.9% after the initial 10 cycles. As can be found that the cathode made of Li3V2(PO4)3/C nanocomposite exhibited the discharge capacities of 116.1, 115.3, 114.4, 114 and 113.8 mA h g−1 for the 100th, 200th, 300th, 400th, 500th cycle, respectively. It is surprising to note that there was less capacity loss even after 500 cycles, which is better than other Li3V2(PO4)3-based materials.44
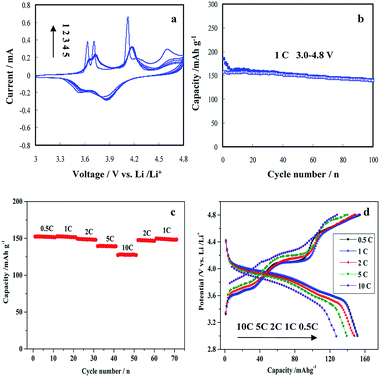
Fig. 5a presents the rate capability of the Li3V2(PO4)3/C nanocomposite from 0.5 to 40C for 10 cycles at each current rate. This material keeps a slightly increasing reversible capacity after each 10th cycle at a high current rate. As can be seen, the Li3V2(PO4)3/C nanocomposite delivered the high discharge capacities of 118, 115, 114, 113, 112 and 110 mA h g−1 at current rates of 0.5, 1, 2, 5, 10 and 20C, respectively. Remarkably, the Li3V2(PO4)3/C nanocomposite had a stable capacity of 106 mA h g−1 even at a current rate as high as 40C, indicating that such a material deliver a high-rate performance. It is noteworthy that the capacity can be restored to its original state even if the current density was returned to 1C after this high-rate measurement. Fig. 5b displays galvanostatic charge–discharge voltage profiles of the Li3V2(PO4)3/C nanocomposite measured at a gradually increased current rate in a potential range of 3.0–4.3 V. The curves for Li3V2(PO4)3/C nanocomposite exhibit three charge–discharge plateaus, which identified as the two-phase transition processes during electrochemical reactions of Li3V2(PO4)3, which agrees well with the CV curves depicted in Fig. 4a. With the increase in charge–discharge current density, Li3V2(PO4)3/C nanocomposite exhibited excellent cycling stability at both high and low current rate. It can be found that the charge–discharge plateaus at a high current rate of 40C are apparent, indicating that Li3V2(PO4)3/C nanocomposite had super high rate performance. It is obvious that the Li3V2(PO4)3 nanocomposite has a small voltage difference of the charge–discharge plateaus and high specific capacities, indicating that Li3V2(PO4)3/C nanocomposite has low electrochemical polarization.45,46 In a word, Li3V2(PO4)3/C nanocomposite exhibited the high capacity and excellent rate capability.
 | ||
| Fig. 5 (a) The rate capacity and (b) charge–discharge curves of Li3V2(PO4)3/C nanocomposite in a potential range of 3.0–4.3 V. | ||
In order to investigate the long-term cycling stability at high rate, we increased the charge–discharge rate to 20C. Fig. 6 shows the long-term cycling performance and Coulombic efficiency of Li3V2(PO4)3/C nanocomposite at a high rate of 20C in a potential range of 3.0–4.3 V. The electrode was cycled at 0.5C for initial 5 cycles and then turned to 20C. As shown in Fig. 6, this material maintained a high Coulombic efficiency, with an average value of 99.1% over 3000 cycles. After 1000 cycles, the electrode retained a high capacity of 105.6 mA h g−1 at a high rate of 20C, and maintained as high as 95.9% of its initial reversible capacity (only 4.1% total capacity loss; ∼0.004% per cycle). Even after 3000 cycles, the Li3V2(PO4)3/C nanocomposite electrode still retained the capacity of 95.1 mA h g−1. An average specific discharge capacity in 3000 cycles at 20C was about 101.8 mA h g−1. The specific capacity and long-term cycling stability for Li3V2(PO4)3/C nanocomposite cathode are better than most of previous reported Li3V2(PO4)3-based cathodes.24,47–49
 | ||
| Fig. 6 The long-term cycling performance and Coulombic efficiency of Li3V2(PO4)3/C nanocomposite at a current rate of 20C in a potential range of 3.0–4.3 V. | ||
The electrochemical performance of Li3V2(PO4)3/C nanocomposite was test in the potential range of 3.0–4.8 V (1C = 198 mA h g−1) for LIBs. Fig. 7a presents the CV curves of Li3V2(PO4)3/C nanocomposite at a scan rate of 0.5 mV s−1 between 3.0 and 4.8 V. As depicted in Fig. 7a, there are four sharp and well-shaped anodic peaks in the first charge curve, corresponding to a sequence of phase transition processes of Li3V2(PO4)3 → Li2.5V2(PO4)3 → Li2V2(PO4)3 → LiV2(PO4)3 → V2(PO4)3. When charged up to 4.8 V, the extraction of the third Li+ will take place. Obviously, the anodic peak current at 4.6 V is the lowest, which is ascribed to the fact that it is difficult to extract the third Li+ in the monoclinic Li3V2(PO4)3. The second and fifth CV curves remained overlapped, indicating the highly reversible performance of Li3V2(PO4)3/C nanocomposite. Fig. 7b shows the cycling performances of Li3V2(PO4)3/C nanocomposite at a current rate of 1C in a potential window of 3.0–4.8 V (1C = 198 mA h g−1). It can be found that the cathode made of Li3V2(PO4)3/C nanocomposite exhibited the discharge capacities of 156.8, 155.1, 149.3 and 139.8 mA h g−1 for the 2nd, 5th, 50th, 100th cycle, respectively. After 100 cycles, the electrode retained a high capacity of 139.8 mA h g−1 at 1C and maintained 89% of its initial reversible capacity, which is better than those of previous results.18,44,49 Fig. 7c and d depict the rate capacity and charge–discharge curves of Li3V2(PO4)3/C nanocomposite in a potential range of 3.0–4.8 V at different current rates between 0.5 and 10C. This material delivered high discharge capacities of 152, 151, 148, 139 and 127 mA h g−1 at 0.5, 1, 2, 5 and 10C, respectively. It is noteworthy that the capacity can be restored to its original state even if the current density was returned to 1C. With increasing current rates, the charge–discharge plateaus became shorter, and the difference in potential between the charging and discharging plateaus increased gradually. However, the charge–discharge plateaus at a high current rate of 10C are apparent, indicating that Li3V2(PO4)3/C nanocomposite has a high rate performance in the potential of 3.0–4.8 V.
The cathode material made of Li3V2(PO4)3/C nanocomposite shows large capacity, high rate performance and excellent long-term cycling stability, which is probably originate from the unique hierarchical architecture. The Li-ions and electrolyte are readily transported in the mesoporous carbon matrix and electrons transport rapidly through the thin carbon layer on the surface of Li3V2(PO4)3 nanoparticles. Such a structure led to a significantly increased electrical conductivity of the overall electrode, resulting in a reduction in the cathode polarization. Moreover, the addition of mesoporous carbon and citric acid led to the small particle size and high degree of crystallinity of Li3V2(PO4)3 during the sintering process, which provides fast Li-ion and electron transport as well as large active surface area. Therefore, the cell made of Li3V2(PO4)3/C nanocomposite can achieve excellent long-term cycling stability, large capacity and high rate capability.
Conclusions
In summary, nanocomposite Li3V2(PO4)3/C was successfully synthesized by combining sol–gel method and nanocasting route. It was found that the size of synthesized particles was only 30–80 nm, which was coated by a thin carbon layer. The Li3V2(PO4)3/C nanocomposite was used as a cathode material in the rechargeable LIBs and exhibited large reversible capacity, high rate performance and excellent long-term cycling stability. For instance, a large reversible capacity of 95 mA h g−1 and an average Coulombic efficiency of 99.1% can be maintained even after 3000 cycles at high rate of 20C in the potential range of 3.0–4.3 V. Moreover, the Li3V2(PO4)3/C nanocomposite delivered a large capacity of 127 mA h g−1 at a high rate of 10C in the voltage range of 3.0–4.8 V. Such excellent properties might be attributed to the unique hierarchical architecture of the Li3V2(PO4)3/C nanocomposite.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (NSFC 21173049).Notes and references
- M. Armand and J.-M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- M. V. Reddy, G. V. Subba Rao and B. V. R. Chowdari, Chem. Rev., 2013, 113, 5364–5457 CrossRef CAS PubMed.
- Y. L. Liang, Z. L. Tao and J. Chen, Adv. Energy Mater., 2012, 2, 742–769 CrossRef CAS PubMed.
- A. K. Padhi, K. S. Nanjndaswamy and J. B. Goodenough, J. Electrochem. Soc., 1997, 144, 1188–1194 CrossRef CAS PubMed.
- S. C. Yin, H. Grondey, P. Strobel, M. Anne and L. F. Nazar, J. Am. Chem. Soc., 2003, 125, 10402–10411 CrossRef CAS PubMed.
- X. Sen, Y. G. Guo and L. J. Wan, Acc. Chem. Res., 2012, 45, 1759–1769 CrossRef PubMed.
- H. J. Yu and H. S. Zhou, J. Phys. Chem. Lett., 2013, 4, 1268–1280 CrossRef CAS.
- Y. S. Hu, Y. G. Guo, R. Dominko, M. Gaberscek, J. Jamnik and J. Maier, Adv. Mater., 2007, 19, 1963–1966 CrossRef CAS PubMed.
- Y. G. Wang, Y. R. Wang, E. Hosono, K. X. Wang and H. S. Zhou, Angew. Chem., Int. Ed., 2008, 47, 7461–7465 CrossRef CAS PubMed.
- I. Moriguchi, R. Hidaka, H. Yamada, T. Kudo, H. Murakami and N. Nakashima, Adv. Mater., 2006, 18, 69–73 CrossRef CAS PubMed.
- J. Li, C. Yue, Y. J. Yu, Y. S. Chui, J. Yin, Z. G. Wu, C. D. Wang, Y. S. Zang, W. Lin, J. T. Li, S. T. Wu and Q. H. Wu, J. Mater. Chem. A, 2013, 1, 14344–14349 CAS.
- P. J. Zhang, L. B. Wang, J. Xie, L. W. Su and C. A. Ma, J. Mater. Chem. A, 2014, 2, 3776–3782 CAS.
- L. X. Zeng, C. Zheng, L. C. Xia, Y. X. Wang and M. D. Wei, J. Mater. Chem. A, 2013, 1, 4293–4299 CAS.
- X. H. Rui, Q. Y. Yan, M. S. Kazacos and T. M. Lim, J. Power Sources, 2014, 258, 19–38 CrossRef CAS PubMed.
- C. Wang, Z. Y. Guo, W. Shen, Q. J. Xu, H. M. Liu and Y. G. Wang, Adv. Funct. Mater., 2014, 21, 5511–5521 CrossRef PubMed.
- M. M. Ren, Z. Zhou, Y. Z. Li, X. P. Gao and J. Yan, J. Power Sources, 2006, 162, 1357–1362 CrossRef CAS PubMed.
- M. M. Ren, Z. Zhou, L. W. Su and X. P. Gao, J. Power Sources, 2009, 189, 786–789 CrossRef CAS PubMed.
- H. D. Liu, P. Gao, J. H. Fang and G. Yang, Chem. Commun., 2011, 47, 9110–9112 RSC.
- A. Q. Pan, J. Liu, J. G. Zhang, W. Xu, G. Z. Cao, Z. M. Nie, B. W. Arey and S. Q. Liang, Electrochem. Commun., 2010, 12, 1674–1677 CrossRef CAS PubMed.
- X. H. Rui, D. H. Sim, K. M. Wong, J. X. Zhu, W. L. Liu, C. Xu, H. T. Tan, N. Xiao, H. H. Hng, T. M. Lim and Q. Y. Yan, J. Power Sources, 2012, 214, 171–177 CrossRef CAS PubMed.
- X. P. Zhang, H. J. Guo, X. H. Li, Z. X. Wang and L. Wu, Electrochim. Acta, 2012, 64, 65–70 CrossRef CAS PubMed.
- C. W. Sun, S. Rajasekhara, Y. Z. Dong and J. B. Goodenough, ACS Appl. Mater. Interfaces, 2011, 3, 3772–3776 CAS.
- G. X. Wang, H. Liu, J. Liu, S. Z. Qiao, G. Q. Lu, P. Munro and H. Ahn, Adv. Mater., 2010, 22, 4944–4948 CrossRef CAS PubMed.
- S. L. Wang, Z. X. Zhang, Z. T. Jiang, A. Deb, L. Yang and S.-I. Hirano, J. Power Sources, 2014, 253, 294–299 CrossRef CAS PubMed.
- X. F. Zhang, K. X. Wang, X. Wei and J. S. Chen, Chem. Mater., 2011, 23, 5290–5292 CrossRef CAS.
- J. Z. Chen, L. Yang, S. H. Fang and S.-I. Hirano, Electrochem. Commun., 2011, 13, 848–851 CrossRef CAS PubMed.
- L. X. Zeng, C. Zheng, J. C. Xi, H. L. Fei and M. D. Wei, Carbon, 2013, 62, 382–388 CrossRef CAS PubMed.
- C. B. Zhu, Y. Yu, L. Gu, K. Weichert and J. Maier, Angew. Chem., Int. Ed., 2011, 50, 6278–6282 CrossRef CAS PubMed.
- J. S. Chen, Y. L. Tan, C. M. Li, Y. L. Cheah, D. Y. Luan, S. Madhavi, F. Y. C. Boey, L. A. Archer and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 6124–6130 CrossRef CAS PubMed.
- L. Q. Mai, S. Li, Y. F. Dong, Y. L. Zhao, Y. Z. Luo and H. M. Xu, Nanoscale, 2013, 5, 4864–4869 RSC.
- A. Q. Pan, D. W. Choi, J. G. Zhang, S. Q. Liang, G. Z. Cao, Z. M. Nie, B. W. Arey and J. Liu, J. Power Sources, 2011, 196, 3646–3649 CrossRef CAS PubMed.
- D. L. Li, M. Tian, R. Xie, Q. Li, X. Y. Fan, L. Gou, P. Zhao, S. L. Ma, Y. X. Shi and H. T. H. Yong, Nanoscale, 2014, 6, 3302–3308 RSC.
- L. X. Zeng, C. Zheng, C. L. Deng, X. K. Ding and M. D. Wei, ACS Appl. Mater. Interfaces, 2013, 5, 2182–2187 CAS.
- X. Ji, K. T. Lee and L. F. Nazar, Nat. Mater., 2009, 8, 500–506 CrossRef CAS PubMed.
- H. S. Zhou, S. M. Zhu, M. Hibino, I. Honma and M. Ichihara, Adv. Mater., 2003, 15, 2107–2111 CrossRef CAS PubMed.
- L. F. Shen, E. Uchaker, C. Z. Yuan, P. Nie, M. Zhang, X. G. Zhang and G. Z. Cao, ACS Appl. Mater. Interfaces, 2012, 4, 2985–2992 CAS.
- F. Han, W. C. Li, M. R. Li and A. H. Lu, J. Mater. Chem., 2012, 22, 9645–9651 RSC.
- L. X. Zeng, F. Y. Xiao, J. C. Wang, S. K. Gao, X. K. Ding and M. D. Wei, J. Mater. Chem., 2012, 22, 14284–14288 RSC.
- A. L. Chen, C. X. Li, R. Tang, L. W. Yin and Y. X. Qi, Phys. Chem. Chem. Phys., 2013, 15, 13601–13610 RSC.
- S. Jun, S. H. Joo, R. Ryoo, M. Kruk, M. Jaroniec, Z. Liu, T. Ohsuna and O. Terasaki, J. Am. Chem. Soc., 2000, 122, 10712–10713 CrossRef CAS.
- L. X. Zeng, Q. F. Li, G. N. Chen, D. P. Tang and M. D. Wei, Electrochim. Acta, 2012, 68, 158–165 CrossRef CAS PubMed.
- W. C. Duan, Z. Hu, K. Zhang, F. Y. Cheng, Z. L. Tao and J. Chen, Nanoscale, 2013, 5, 6485–6490 RSC.
- C. Wang, H. M. Liu and W. S. Yang, J. Mater. Chem., 2012, 22, 5281–5285 RSC.
- F. Teng, Z. H. Hu, X. H. Ma, L. C. Zhang, C. X. Ding, Y. Yu and C. H. Chen, Electrochim. Acta, 2013, 91, 43–49 CrossRef CAS PubMed.
- J. Su, X. L. Wu, J. S. Lee, J. Kim and Y. G. Guo, J. Mater. Chem. A, 2013, 1, 2508–2514 CAS.
- L. Zhang, H. F. Xiang, Z. Li and H. H. Wang, J. Power Sources, 2012, 203, 121–125 CrossRef CAS PubMed.
- Q. L. Wei, Q. Y. An, D. D. Chen, L. Q. Mai, S. Y. Chen, Y. L. Zhao, K. M. Hercule, L. Xu, A. M. Khan and Q. J. Zhang, Nano Lett., 2014, 14, 1042–1048 CrossRef CAS PubMed.
- Y. Q. Qiao, X. L. Wang, Y. J. Mai, J. Y. Xiang, D. Zhang, C. D. Gu and J. P. Tu, J. Power Sources, 2011, 196, 8706–8709 CrossRef CAS PubMed.
- J. T. Xu, S. L. Chou, C. F. Zhou, Q. F. Gu, H. K. Liu and S. X. Dou, J. Power Sources, 2014, 246, 124–131 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |