A novel Pd/C-catalyzed conversion of glucose to 1,2-propanediol by water splitting with Zn†
Jie Wanga,
Guodong Yaoa,
Yuanqing Wangc,
Hua Zhanga,
Zhibao Huoa and
Fangming Jin*ab
aSchool of Environmental Science and Engineering, State Key Lab of Metal Matrix Composites, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, China. E-mail: fmjin@sjtu.edu.cn; Fax: +86 21-54742283; Tel: +86-21-54742283
bGraduate School of Environmental Studies, Tohoku University, Japan
cNakamura Laboratory, RIKEN Innovation Center, 2-1 Hirosawa, Wako, Saintama 351-0198, Japan
First published on 3rd June 2015
Abstract
A novel Pd/C-catalysed conversion of glucose to 1,2-propanediol by water splitting with Zn was proposed. The yield of 1,2-akanediols reached 48%, mainly including 1,2-propanediol (33.3%), the reported highest yield from glucose. Water, as a solvent, provides an in situ and active hydrogen source. The formed ZnO acts as a catalyst with Pd/C.
With increasing environmental awareness and global energy crises, effective methods of converting renewable biomass into valuable chemicals have gathered much attention.1–5 1,2-Propanediol (1,2-PD) is an important chemical, widely used in drugs, antifreeze and the production of polyesters and polyethers. However, 1,2-PD is mainly produced from petroleum-derived feedstocks. Thus, an efficient conversion of renewable biomass like carbohydrates, and their derivatives such as lactic acid and glycerol, to 1,2-PD is highly desirable. Although there have been many reports involving the conversion of carbohydrates,6–10 lactic acid11,12 and glycerol13–16 into 1,2-PD, gaseous hydrogen, mainly coming from fossil fuels, and complicated-designed bifunctional catalysts such as CuCr,6 Ni–Cu/ZnO,7 WO3/Al2O3 + AC,8 Pt–PEI,9 Ni–W2C,10 Ru/TiO2,11 Ru–MoOx/C,12 Ag–OMS-2,13 Cu/MOx,14 CuNP@AlOx15 and Ni3P16 are usually needed. Moreover, the storage and transportation of gaseous hydrogen are considered as energy-intensive. Therefore, developing a new method for the hydrogenation of glucose with alternative hydrogen sources and simple catalysts is urgently required.
Water is the most abundant hydrogen resource in the earth. If hydrogen from water splitting by solar energy can be in situ used for the hydrogenation of biomass into chemicals, it will be a promising method to solve the energy crisis. However, it is extremely challenging. In contrast to the direct use of solar energy, an integrated technology can be expected to have a high potential for the hydrogenation of biomass into chemical by water splitting with solar energy. Our previous research has demonstrated that the hydrogen can be easily produced with commercially available and non-precious metals such as Zn, Fe or Al as a reductant metal under hydrothermal conditions and used for the reduction of CO2,17–21 formic acid22 and glycolide.23 Moreover, the metal oxides, such as ZnO and Fe2O3, have been reported to be dissociated to metals and O2 with solar energy.24–26 Thus, an efficient conversion of biomass to value-added chemicals with solar energy can be achieved by combining hydrothermal method to the conversion of biomass into chemicals with metals and solar reduction of metal oxides into metals. Thus, we turned our attention to the much more challenging hydrogenation of biomass into value-added chemicals with metals as a reductant in water. Herein, we present an efficient Pd/C-catalyzed conversion of glucose to 1,2-PD by water splitting with Zn (eqn (1)).
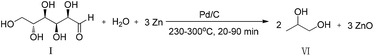 | (1) |
First, the conversion of glucose into 1,2-PD was examined with different metals as a reductant at 250 °C for 30 min. The reaction did not give the desired 1,2-PD when using only Zn or Pd/C (Table 1, entry 1 and 2), respectively. Interestingly, however, the formation of 1,2-PD was clearly observed in the presence of Zn and Pd/C together (Table 1, entry 3), and the yield of 1,2-PD can reach 22.4% (based on the mole of carbon in the product and reactant). In this case, a little amount of ethylene glycol (EG) and 1,2-butanediol (1,2-BD) were also observed. The analysis of solid residuals after the reactions by XRD showed that only ZnO and no other peaks were observed, indicating that Zn was oxidized to ZnO (see Fig. SI-5†), and the hydrogen for converting glucose to 1,2-PD came from water by the oxidation of Zn. Reactions with other metals such as Al and Fe as reductants were also conducted. Results showed that no noticeable 1,2-PD was detected (Table 1, entries 5 and 6). Further, metal oxides such as CuO and Ni2O3 as catalysts were investigated in the presence of Zn. It was found that the yields of 1,2-PD were quite low, only 8.7% and 9.7% (Table 1, entry 7 and 8). These results suggest that only Zn as a reductant and Pd/C as a catalyst are effective for the conversion of glucose to 1,2-PD.
| Entry | Reductant | Catalyst | Yielde (%) | ||
|---|---|---|---|---|---|
| 1,2-PGb | EGb | 1,2-BDb | |||
| a Typical reaction conditions: 200 mg (1.11 mmol) glucose, 450 mg (6.9 mmol) Zn, 35 mg (0.016 mmol) Pd/C, water filling: 50%, 250 °C and 30 min.b EG and 1,2-PG, and 1,2-BD are abbreviations of ethylene glycol, 1,2-propylene glycol and 1,2-butanediol respectively.c 588 mg (9 mmol) Zn and 65 mg (0.03 mmol) Pd/C.d The Pd/C was reused.e The conversion of glucose is near to 100%. | |||||
| 1 | Zn | No | 0.22 | None | None |
| 2 | No | Pd/C (35 mg) | None | None | None |
| 3 | Zn | Pd/C (35 mg) | 22.47 | 3.55 | 3.8 |
| 4 | Znc | Pd/C (65 mg) | 33.3 | 7.1 | 7.6 |
| 5 | Alc | Pd/C (65 mg) | None | None | None |
| 6 | Fec | Pd/C (65 mg) | 1.8 | None | None |
| 7 | Zn | CuO (100 mg) | 9.7 | None | None |
| 8 | Zn | Ni2O3 (100 mg) | 8.7 | None | None |
| 9 | Zn | Pd/Cd (35 mg) | 9.3 | None | None |
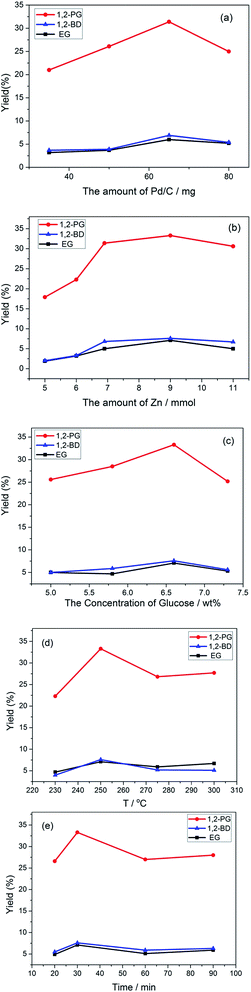
After confirming the activity of Zn and Pd/C for the conversion of glucose into 1,2-PD, we investigated the characteristics of the reaction to find the optimum condition. First, the effects of initial amounts of Pd/C and Zn were studied at 250 °C, 30 min. As shown in Fig. 1(a), the initial amount of Pd/C strongly affected the 1,2-PD yield at a fixed amount of Zn (451 mg, 6.9 mmol). The yield of 1,2-PD first increased and then decreased with the increase in the amount of Pd/C, and the highest yield (31.4%) occurred at 65 mg, 0.03 mmol Pd/C. Overmuch Pd/C may result in the decomposition of 1,2-PD. Then, the effect of the amount of Zn was investigated by fixing the amount of Pd/C at 65 mg, 0.03 mmol. The yield of 1,2-PD greatly increased from 17.9% to 33.3% when the amount of Zn increased from 5 mmol to 9 mmol. Further increasing the amount of Zn to 11 mmol did not give a higher yield of 1,2-PD. The increase in the yield of 1,2-PD with the increase in the amount of Zn most likely occurred because stronger reduction conditions or a larger amount of hydrogen improves the conversion of glucose into 1,2-PD. These results indicate that the amount of Zn and Pd/C has a great effect on the yield of 1,2-PD. Moreover, EG and 1,2-BD were also detected and analyzed quantitatively in all experiments. As shown in Fig. 1, the yields of EG and 1,2-BDwere low (about 5% to 7%) and did not change a lot with the increase in the amount of Pd/C and Zn, which indicated that the formation of EG and 1,2-BD underwent a quite different reaction pathway with the production of 1,2-PD.
The previous research involving the conversion of glucose to 1,2-PD has reported that a higher initial concentration of glucose was not favored for the conversion of glucose into 1,2-PD and usually limited to a low concentration, such as 0.25 wt% (ref. 8) with 14.1% yield of 1,2-propnediol and 1 wt% (ref. 27) with 25.6% yield of 1,2-PD. For an industrial application, a high initial concentration of glucose is desired. Thus, we examined the effect of the initial concentration of glucose. As a result, the yield of 1,2-PD increased with the increase in the initial amount of glucose and reached a climax when the concentration of glucose got up to 6.6 wt% (see Fig. 1(c)), which is much higher than that reported previously.8,27 Further increase in the initial glucose concentration led to decrease in the yield, which could be due to the polymerization of glucose as a competitive side reaction.
Finally, the effects of the temperature and time on the yield of 1,2-PD were investigated (Fig. 1(d) and (e)). The yield of 1,2-PD greatly increased when the temperature increased from 230 °C to 250 °C (Fig. 1(d)). Then, the yield of 1,2-PD dropped slightly and remained nearly constant when the reaction temperature further increased to 300 °C. The observed decrease at over 250 °C might result from the further decomposition of 1,2-PD. The time profile indicated that an increase in the reaction time from 20 min to 30 min led to a great increase in the yield of 1,2-PD (Fig. 1(e)), however, a longer reaction time than 30 min led to a decrease in the 1,2-PD yield slightly, which might be due to the decomposition of 1,2-PD. Similarly, EG and 1,2-BD were also detected and analyzed quantitatively at all temperatures and reaction times. As shown in Fig. 1(d) and (e), all yields were very low and did not change a lot with the change of temperature and time.
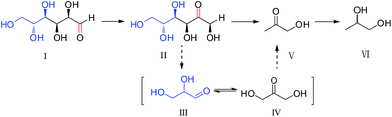
To investigate the reaction pathways, we examined the intermediate products and their distribution at different time. As showed in Fig. SI-2,† product within 2 min reactions detected by GC-MS was mainly acetol. With increasing the reaction time, 1,2-PD, EG and 1,2-BD became the main products. The products analyzed by HPLC showed that fructose formed as an intermediate product (see Fig. SI-4†). Then, the variation of the main products with the reaction time was examined. As shown in Fig. 2(a), the amount of glucose (I) sharply decreased, while fructose (II) increased from 20 s to 40 s, indicating that the isomerisation of glucose to fructose occurred mainly at the beginning of the reaction. After 40 s, fructose (II) decreased, while acetol (V) gradually increased, suggesting that fructose was further converted into acetol. After 2 min, acetol decreased gradually (Fig. 2(b)), and the amount of 1,2-PD (VI) greatly increased, indicating that acetol further was converted into 1,2-PD finally. In addition, EG and 1,2-BD were also detected as by-products, and no significant change in their concentrations was observed with the change in the reaction time. In the conversion of glucose to 1,2-PD, a reaction pathway (including isomerisation of glucose, cleavage of fructose by the retro-aldol reaction, and hydrogenation of acetol) has been proposed. And dihydroxyacetone (IV) and glyceraldehydes (III) are considered to be intermediates via the cleavage of fructose by the retro-aldol reaction of fructose.7,9 However, dihydroxyacetone (IV) and glyceraldehydes (III) were not detected in this study, probably due to their rapid hydrogenation to acetol under hydrothermal conditions.
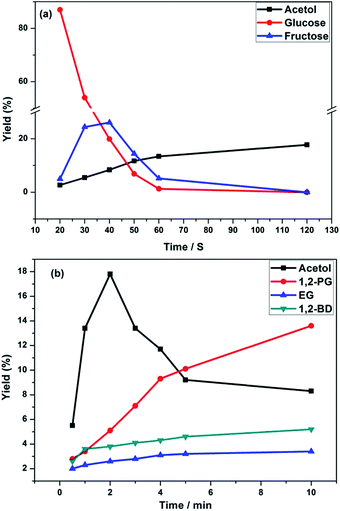 | ||
| Fig. 2 Products distribution from glucose transformation (a) 20 s to 2 min; (b) 30 s to 10 min over 35 mg 5% Pd/C, 6.9 mmol Zn at 250 °C. | ||
Based on the detected intermediate products and their distribution with the change in reaction time, a reaction pathway from glucose to 1,2-PD is proposed as shown in Scheme 1. At the beginning, glucose isomerizes to fructose, which then undergoes the retro-aldol reaction to dihydroxyacetone and glyceraldehydes with the cleavage of C–C bond. Then, the dihydroxyacetone and glyceraldehydes are reduced to acetol. Finally, the acetol is further reduced to 1,2-PD.
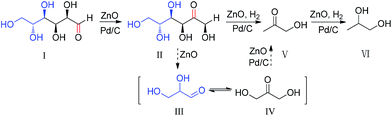
After proposing the reaction pathway, we investigated catalytic activity of Pd/C as well as ZnO in different stages from glucose to 1,2-PD as shown in Scheme 1, respectively. Catalytic activity of ZnO was examined because ZnO is formed when using Zn as a reductant for producing hydrogen. Pd/C was first examined in the first stage reaction (the isomerisation of glucose to fructose). As shown in Table 2, 25.3% fructose was achieved in the presence of Pd/C (entry 1), while only 13.4% fructose was produced in the absence of Pd/C (entry 2). Meanwhile, the remaining glucose (39.7%) in the presence of Pd/C was much lower than that (74%) in the absence of Pd/C. Thus, it is obvious that Pd/C catalyzed not only the isomerisation of glucose to fructose but also the decomposition of glucose. Then, the experiments with the addition of ZnO were conducted to test whether ZnO formed plays a catalyst role in the isomerisation of glucose to fructose. Compared to the blank experiment, in which the yields of fructose and glucose were 13.4% and 74%, respectively, 32.6% fructose was obtained and 63.9% glucose was remaining (Table 2, entry 3), which shows that the presence of ZnO greatly accelerated the isomerisation of glucose to fructose; however, ZnO has no significant effect on the decomposition of glucose. Even the amount of ZnO was decreased to 27 mg, 0.33 mmol (30% of the amount of glucose), the catalytic effect was observed (Table 2, entry 4). Further, the effect of reaction time on the yield of fructose from glucose in the presence of ZnO was investigated to further examine the catalytic activity of ZnO in the isomerisation of glucose into fructose. The yield of fructose gradually increased in the first 50 s, and then decreased, while glucose decreased for all the reaction time (see Fig. SI-7†), which further confirms the catalytic activity of ZnO in the isomerisation of glucose into fructose. Thus, it is clear that both Pd/C and ZnO catalyze the isomerisation of glucose to fructose.
| Entry | Feedstock | Additive | Yield (%) | ||
|---|---|---|---|---|---|
| Glucose | Fructose | Acetol | |||
| a Reaction condition: Zn or ZnCl2: 6.9 mmol; Pd/C: 35 mg, 0.016 mmol; glucose: 1.11 mmol; H2O: 2.85 ml; 250 °C; 60 s.b ZnO: 1.38 mmol; 30 s.c 6 MPa H2 was added.d ND: not determined.e ZnO: 0.33 mmol; 30 s. | |||||
| 1 | Glucose | Pd/C | 39.7 | 25.3 | NDd |
| 2 | Glucose | Blank | 74 | 13.4 | ND |
| 3 | Glucose | ZnOb | 63.9 | 32.6 | ND |
| 4 | Glucose | ZnOe | 81.2 | 18.7 | ND |
| 5 | Fructose | Zn | ND | ND | 4.7 |
| 6 | Fructose | ZnOb | ND | ND | 4.5 |
| 7 | Fructose | Pd/C | ND | ND | 2.5 |
| 8 | Fructose | ZnCl2 | ND | ND | 2.5 |
| 9 | Fructose | Blank | ND | ND | 2.8 |
| 10 | Fructose | Pd/C + Zn | ND | ND | 13.5 |
| 11 | Fructose | ZnO + Pd/C + H2c | ND | ND | 8.3 |
For the second stage of the reaction (from fructose to acetol), the possible catalytic roles of Zn, ZnO, ZnCl2 and Pd/C were investigated in the C–C cleavage of fructose and the reduction of acetol, respectively. The acetol yield with Zn (4.7%) or ZnO (4.2%) was clearly higher than that with Pd/C (2.5%) or with ZnCl2 (2.5%) or without any additive (2.6%) (Table 2, entry 5–9), indicating that ZnO act as a catalyst in the selective cleavage of C–C in fructose to dihydroxyacetone and glyceraldehydes. However, the addition of Pd/C and Zn greatly improved the yield of acetol (Table 2, entry 10) and 13.5% yield of acetol could be achieved, which was much higher than others. Thus, it is obvious that ZnO can efficiently catalyze the selective cleavage of C–C, and both of Pd/C and ZnO can catalyze the reduction of dihydroxyacetone and glyceraldehydes to acetol.
The in situ hydrogen produced in water with Zn may be different from the gaseous hydrogen (H2). To test this assumption, a reaction by the replacement of Zn with gaseous hydrogen and ZnO was conducted. The yield of acetol with gaseous hydrogen in the presence of ZnO and Pd/C was 8.3% (Table 2, entry 11), which was lower than the yield (13.4%) with Zn and Pd/C. These results show that the activity of the in situ-formed hydrogen is much higher than that of gaseous hydrogen.
As for the last step (from acetol to 1,2-PD), acetol was used as a feedstock. As shown in Table 3, the yield of 1,2-PD was as high as 98% when using Zn and Pd/C (entry 1), while the yield of 1,2-PD was only 18.9% in the absence of Pd/C (entry 2). Thus, Pd/C could catalyze the reduction of acetol to 1,2-PD. Further, reactions with gaseous hydrogen were also examined to test the difference between the gaseous hydrogen and the in situ-formed hydrogen. As a result, the yield of 1,2-PD from acetol was 40.2% with gaseous hydrogen in the presence of ZnO (Table 3, entry 3) and 55% even after 2 h (see Fig. SI-6†). Further, an experiment without ZnO was also conducted. As a result, only 4.1% of 1,2-PD was achieved (Table 3, entry 4), suggesting that ZnO acts as a catalyst in the reduction of acetol to 1,2-PD. An experiment only with ZnO provided 9.8% yield of 1,2-PD. From these results, ZnO and Pd/C act a synergetic catalytic role in the reduction of acetol to 1,2-PD. Finally, the effect of gaseous hydrogen was also investigated on the yield of 1,2-PD with glucose as a feedstock. The yield of 1,2-PD was only 2.9% (Table 3, entry 6).
| Entry | Reactant | Reductant | Catalyst | Yield (%) |
|---|---|---|---|---|
| a Typical reaction conditions: 1.11 mmol reactant, 6.9 mmol Zn, 35 mg, 0.016 mmol Pd/C, water filling: 50%, 250 °C and 30 min.b The optimized condition: 1.11 mmol reactant, 9 mmol Zn and 65 mg, 0.03 mmol Pd/C, water filling: 50%, 250 °C and 30 min.c 6 MPa H2 was added. | ||||
| 1 | Acetol | Zn | 5% Pd/C | 98 |
| 2 | Acetol | Zn | No | 18.9 |
| 3 | Acetolc | H2 | ZnO (6.9 mmol) + 5% Pd/C (35 mg) | 40.2 |
| 4 | Acetolc | H2 | 5% Pd/C (35 mg) | 4.1 |
| 5 | Acetolc | H2 | ZnO (6.9 mmol) | 9.8 |
| 6 | Glucosec | H2 | ZnO (6.9 mmol) + 5%Pd/C (35 mg) | 2.9 |
| 7 | Fructoseb | Zn | 5% Pd/C | 35.5 |
According to discussion above and the reaction pathway showed in Scheme 1, a more detailed reaction pathway with catalysts can be proposed in Scheme 2.
In order to figure out the key step of the whole reactions for the selectivity of 1,2-PD, the yields of 1,2-PD from fructose and glucose were compared. The yield of 1,2-PD from fructose (35.5%) was slightly higher than the yield from glucose (Table 3, entry 7), indicating that there is no significant difference with glucose and fructose as a feedstock. In other word, the glucose can be converted into fructose completely and the yield of 1,2-PD was not limited by the isomerisation of glucose. For the reaction with acetol as a feedstock, surprisingly, the yield of 1,2-PD was quite high, up to 98% (see Table 3, entry 1), suggesting that the almost acetol produced could be converted into 1,2-propandiol. Thus, the reduction of fructose to acetol determines the selectivity of 1,2-PD. If the selectivity of fructose to acetol could be improved, then a higher yield of 1,2-PD should be achieved. The study with this option is in progress.
In addition, the liquid and gas sample after the reaction were analyzed by TOC (total organic carbon) and GC/TCD respectively to investigate the carbon balance. The amount of carbon in the liquid sample was about 83.6% of the glucose added, which was higher than the total yields of 1,2-PD, EG, and 1,2-BD. This can be attributed to the formation of by-products (see Fig. SI-2†) and the possible side reactions were provided (see Scheme SI-1†). Moreover, approximate 3.9% of CO2 were detected by the GC/TCD analysis for the gas sample (see Fig. SI-8†). Thus, the TOC of the liquid and gas sample was 87.5%, which still can not balance the carbon between the consumed glucose and the detected products. Lund et al.,28 have reported that humins with 5-hydroxymethylfurfural as a reactant derived from glucose might form under hydrothermal conditions, and thus, we suggest that humins also was formed in our system and leaded to the difference between the total carbon (87.5%), detected from the liquid and gas sample, and the consumed glucose (100%). Also the activity and stability of Pd/C was investigated, the activity of Pd/C has a clear decrease after the first time (Table 1, entry 12). Further investigation of the catalytic activity and stability is now in progress.
Conclusions
We have demonstrated for the first time that the conversion of glucose to 1,2-alkanediols can be realized by water splitting with Zn and Pd/C. Not only did Zn provide the active hydrogen by water splitting, but also the formed ZnO act as a catalyst, especially for the C–C cleavage of fructose. Pd/C and ZnO synergistically catalyze the isomerisation of glucose and the reduction of acetol. The present study is helpful to provide a promising method for efficiently converting carbohydrate to 1,2-PD with solar energy by combining with the reduction of ZnO using solar energy.Acknowledgements
The authors thank the financial support of the National Natural Science Foundation of China (no. 21277091 & no. 51472159), the State Key Program of National Natural Science Foundation of China (no. 21436007), Key Basic Research Projects of Science, Technology Commission of Shanghai (no. 14JC1403100) and China Postdoctoral Science Foundation (no. 2013M541520). We gratefully acknowledge financial support from the state key laboratory of coal-based low carbon energy, ENN.Notes and references
- Y. Liu, L. Chen, T. Wang, Y. Xu, Q. Zhang, L. Ma, Y. Liao and N. Shi, RSC Adv., 2014, 4, 52402–52409 RSC.
- X. Liu, X. Wang, S. Yao, Y. Jiang, J. Guan and X. Mu, RSC Adv., 2014, 4, 49501–49520 RSC.
- J. Song, H. Fan, J. Ma and B. Han, Green Chem., 2013, 15, 2619–2635 RSC.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- S. I. George, W. Huber and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef PubMed.
- Z. Xiao, S. Jin, M. Pang and C. Liang, Green Chem., 2013, 15, 891 RSC.
- X. Wang, L. Meng, F. Wu, Y. Jiang, L. Wang and X. Mu, Green Chem., 2012, 14, 758 RSC.
- Y. Liu, C. Luo and H. Liu, Angew. Chem., 2012, 51, 3249–3253 CrossRef CAS PubMed.
- Y. Kanie, K. Akiyama and M. Iwamoto, Catal. Today, 2011, 178, 58–63 CrossRef CAS PubMed.
- M. Y. Zheng, A. Q. Wang, N. Ji, J. F. Pang, X. D. Wang and T. Zhang, ChemSusChem, 2010, 3, 63–66 CrossRef CAS PubMed.
- A. Primo, P. Concepcion and A. Corma, Chem. Commun., 2011, 47, 3613–3615 RSC.
- Y. Takeda, T. Shoji, H. Watanabe, M. Tamura, Y. Nakagawa, K. Okumura and K. Tomishige, ChemSusChem, 2015, 8, 1170–1178 CrossRef CAS PubMed.
- G. D. Yadav, P. A. Chandan and D. P. Tekale, Ind. Eng. Chem. Res., 2012, 51, 1549–1562 CrossRef CAS.
- Y. Feng, H. Yin, L. Shen, A. Wang, Y. Shen and T. Jiang, Chem. Eng. Technol., 2013, 36, 73–82 CrossRef CAS PubMed.
- T. Mizugaki, R. Arundhathi, T. Mitsudome, K. Jitsukawa and K. Kaneda, Chem. Lett., 2013, 42, 729–731 CrossRef CAS.
- G. Shi, L. Su and K. Jin, Catal. Commun., 2015, 59, 180–183 CrossRef CAS PubMed.
- L. Lyu, F. Jin, H. Zhong, H. Chen and G. Yao, RSC Adv., 2015, 5, 31450–31453 RSC.
- F. Jin, X. Zeng, J. Liu, Y. Jin, L. Wang, H. Zhong, G. Yao and Z. Huo, Sci. Rep., 2014, 4, 4503 Search PubMed.
- Z. Huo, M. Hu, X. Zeng, J. Yun and F. Jin, Catal. Today, 2012, 194, 25–29 CrossRef CAS PubMed.
- F. Jin, Y. Gao, Y. Jin, Y. Zhang, J. Cao, Z. Wei and R. L. Smith Jr, Energy Environ. Sci., 2011, 4, 881–884 CAS.
- B. Wu, Y. Gao, F. Jin, J. Cao, Y. Du and Y. Zhang, Catal. Today, 2009, 148, 405–410 CrossRef CAS PubMed.
- J. Liu, X. Zeng, M. Cheng, J. Yun, Q. Li, Z. Jing and F. Jin, Bioresour. Technol., 2012, 114, 658–662 CrossRef CAS PubMed.
- L. Xu, Z. Huo, J. Fu and F. Jin, Chem. Commun., 2014, 50, 6009–6012 RSC.
- A. Steinfeld, Int. J. Hydrogen Energy, 2002, 27, 611–619 CrossRef CAS.
- S. M. P. Haueter, R. Palumbo and A. Steinfeld, Sol. Energy, 1999, 67, 161–167 CrossRef.
- A. S. A. Weidenkaff, A. Wokaun, P. O. Auer, B. Eichler and A. Reller, Sol. Energy, 1999, 65, 59–69 CrossRef CAS.
- X. Wang, F. Wu, S. Yao, Y. Jiang, J. Guan and X. Mu, Chem. Lett., 2012, 41, 476–478 CrossRef CAS.
- S. K. R. Patil and C. R. F. Lund, Energy Fuels, 2011, 25, 4745–4755 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization data. See DOI: 10.1039/c5ra08482b |
| This journal is © The Royal Society of Chemistry 2015 |