Solution-processed indium-zinc-oxide thin-film transistors based on anodized aluminum oxide gate insulator modified with zirconium oxide
Yuzhi Li,
Linfeng Lan*,
Peng Xiao,
Zhenguo Lin,
Sheng Sun,
Wei Song,
Erlong Song,
Peixiong Gao,
Dan Wang*,
Honglong Ning and
Junbiao Peng*
State Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou 510640, China. E-mail: lanlinfeng@scut.edu.cn; wangdan@scut.edu.cn; psjbpeng@scut.edu.cn
First published on 5th June 2015
Abstract
Solution-processed indium-zinc-oxide (IZO) thin-film transistors (TFTs) based on anodized aluminum oxide gate insulator modified with a zirconium oxide (ZrOx) interlayer were fabricated. By introduction of the ZrOx interlayer, the IZO-TFTs exhibited improved performance with a higher mobility of 7.8 cm2 V−1 s−1, a lower Vth of 4.6 V and a lower SS of 0.21 V dec−1 compared to those without the ZrOx interlayer. Comprehensive studies showed that the Al element easily diffused into the IZO film and formed AlOx clusters which acted as defects to deteriorate TFT performance; and after modification with a ZrOx interlayer, the diffusion of Al was suppressed and the Zr diffusing effect almost could be ignored. These results suggested that the introduction of an interlayer with less diffusing effect as well as an effect of blocking the elements from the gate insulator diffusing into the channel layer could be an effective way to improve the electrical performance for solution-processed oxide TFTs.
1 Introduction
Thin-film transistors (TFTs) based on amorphous oxide semiconductors (AOSs), as promising candidates for unit pixel drivers in active-matrix organic light-emitting diode displays (AMOLEDs) and liquid-crystal displays (LCDs), have attracted a great amount of attention for their competitive performances, including high field-effect mobility, good optical transparency and good processing compatibility with a TFT production line using conventional amorphous Si (a-Si) as a channel material.1,2 Over the last decade, a lot of excellent-performance AOSs, such as indium-gallium-zinc oxide (IGZO), indium-zinc oxide (IZO), zinc-tin oxide (ZTO), and indium-zinc-tin oxide (IZTO), have been reported and intensively studied.3–7 Although the intrinsic properties and quality of AOS thin films are the most important factors determining the electrical properties of TFTs, the gate insulators also play an important role due to the interface coupling between the gate insulator and the AOS.8,9 In our previous reports, the high performance IGZO- and IZO-TFTs were successfully fabricated using anodized AlOx to replace SiO2 as insulators.10–12 The low leakage current and relatively high dielectric constant make the anodized AlOx attractive for the gate insulators of the TFTs. However, the AOS thin films were manufactured by vacuum-based method, which is a major obstacle for further cutting down the fabricated cost. Instead, solution-processed AOS-TFTs are more attractive because of the low-cost and large-area process.13–16Herein, solution-processed IZO-TFTs with anodized AlOx gate insulator were fabricated. The surface of the anodized AlOx was modified with a thin solution-processed ZrOx interlayer. The IZO-TFTs with ZrOx/AlOx gate insulator show improved performance compared with IZO-TFTs with AlOx gate insulator. The mechanisms for the performance improvement were studied in detailed.
2 Experiments and characterization
To prepare the ZrOx precursor solution, 0.2 M zirconium oxychloride hydrate (ZrOCl2·8H2O) and 0.5 mL aqueous hydrogen peroxide solution (30 wt%) were added into 4.5 mL 2-methoxyethanol. The solution was stirred vigorously for 4 h to form a transparent solution. For IZO precursor solution, 0.1 M metal precursor solution was prepared by adding indium nitrate hydrate [In(NO3)3·4.5H2O] and zinc nitrate hydrate [Zn(CH3COO)2·2H2O] into 2-methoxyethanol with molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The solution was stirred at 60 °C for 12 h. Meanwhile, a small amount of monoethanolamine was added to the mixture as stabilizer after the dissolution of the metal salts. Both solutions were filtered through a 0.22 μm syringe filter before spin-coating.
2. The solution was stirred at 60 °C for 12 h. Meanwhile, a small amount of monoethanolamine was added to the mixture as stabilizer after the dissolution of the metal salts. Both solutions were filtered through a 0.22 μm syringe filter before spin-coating.
The IZO-TFTs based on ZrOx/AlOx bilayer insulator were prepared with the bottom-gate, top-contact configuration, as shown in Fig. 1(a). An aluminium gate electrode was firstly deposited on a glass with a thickness of 300 nm. Then, a 190 nm thick anodic AlOx layer was formed on the surface of the gate electrode by anodic oxidation technology. The details of anodic oxidation process have ever been reported elsewhere.10 Then, the ZrOx precursor solution was spin-coated at 3000 rpm for 30 s on anodic AlOx layers that were treated by oxygen plasma for 20 min. The ZrOx thin films were firstly soft-baked at 200 °C for 5 min and then hard-baked at 350 °C for 2 h under ambient condition. The IZO precursor solution was spin-coated on ZrOx at 3000 rpm for 30 s in air. To minimize fringing current effects and gate leakage, the IZO layer was patterned by removing the films outside the channel area with a mechanically-controlled probe tip. This tip was dragged around each transistor, as close as possible to the channel area, prior to the annealing process.17 After that, the IZO thin film was annealed at 120 °C for 10 min and then annealed at 350 °C for 1 h under air atmosphere. Aluminum thin films (250 nm of thickness), as source and drain electrodes (S/D), were finally deposited on IZO layers by thermal evaporation and patterned using a metal shadow mask. The width (W) and length (L) of the channel was 1000 μm and 300 μm, respectively. For the IZO-TFTs based on anodic AlOx signal layer insulators, the IZO thin films were directly spin-coated on the anodic AlOx layers without ZrOx layers, and other processes were all the same as those based on anodic ZrOx/AlOx bilayer insulators.
X-ray photoelectron spectroscopy (XPS, Kratos, Axis Ultra DLD) using monochromatic Al Kα radiation (∼1486.6 eV) was used to examine chemical composition of the oxide films. The XPS data were calibrated with C 1s peak at ∼284.6 eV. The thickness and structure of the oxide thin films were characterized by transmission electron microscopy (TEM, FEI Titan Themis 200) equipped with an energy dispersive X-ray spectrometer (EDS). The electrical measurements were performed using a semiconductor parameter analyzer (Agilent 4155C) in air.
3 Results and discussion
Fig. 1(b) and (c) display scanning transmission electron microscopy (STEM) image of the IZO/ZrOx/AlOx/Al cross-sectional structure and high-resolution transmission electron microscopy (HR-TEM) image of IZO/ZrOx/AlOx cross-section, respectively. Both images reveal a uniform and continuous ZrOx/AlOx interface without pinholes or hillocks. The thicknesses of Al, AlOx, ZrOx, and IZO were measured to be 130, 190, 14, and 8 nm, respectively, which were closed to those measured with ellipsometer or step profiler. Fig. 1(d) shows the fast Fourier transform (FFT) patterns for different areas indicated in Fig. 1(c), revealing the presence of nanocrystalline IZO, ZrOx, and AlOx domains, and the ZrOx layer had the highest crystallinity.Fig. 2(a) and (b) show the output characteristics obtained from IZO-TFTs with and without ZrOx interlayer, respectively. It could be seen clearly that both TFTs showed typical n-channel, saturated characteristics, and the IZO-TFT with ZrOx interlayer had higher output drain current (ID). There were no current crowding effects in the low drain voltage (VD) regimes, indicating that both devices had good contacts between S/D and channel layer. Fig. 2(c) shows the transfer characteristics and the corresponding gate leakage current (IG) versus gate voltage (VG) of the IZO-TFTs with and without ZrOx interlayer. The IZO-TFT without ZrOx interlayer exhibited a field-effect mobility (μ) of 2.8 cm2 V−1 s−1, a threshold voltage (Vth) of 4.9 V and a sub-threshold slope (SS) of 0.33 V dec−1, while the IZO-TFT with ZrOx interlayer showed a much better performance with a higher μ of 7.8 cm2 V−1 s−1, a lower Vth of 4.6 V and a lower SS of 0.21 V dec−1. The IG–VG curves showed that the leakage current reduced slightly after inserted with ZrOx layer. The electrical properties of both devices are summarized in Table 1. It was worth noting that the hysteresis of the transfer curves between forward and reverse sweeps was reduced for IZO-TFTs with ZrOx interlayer, suggesting that there are fewer shallow traps which can be trapped or release under positive or negative gate voltage. To evaluate the total trap density (Nt), including the semiconductor bulk (Nb) and semiconductor–insulator interfacial traps (Di), Nt was calculated from the SS values,18
 | (1) |
| Nt = Di + tNb | (2) |
| Insulator | Mobility (cm2 V−1 s−1) | Vth (V) | Ion/Ioff | SS (V dec−1) |
|---|---|---|---|---|
| Without ZrOx | 2.8 | 4.9 | 4.8 × 106 | 0.33 |
| With ZrOx | 7.8 | 4.6 | 3.5 × 106 | 0.21 |
To investigate how the ZrOx interlayer affect the total traps, the samples were characterized by high-angle annular dark-field (HAADF) STEM and EDS. Fig. 3 shows the maps of the elemental distribution of IZO/AlOx/Al/glass and IZO/ZrOx/AlOx/Al/glass, respectively, revealing good consistency with the STEM images and uniform distributions for all the elements in each layer. It can be seen clearly that there were Al elements distributing in the whole IZO film for the sample without ZrOx interlayer, while much fewer Al elements were found in the IZO film for the sample with ZrOx interlayer. Therefore, the ZrOx interlayer has an effect of blocking Al elements from diffusing into IZO film, which was supported by the quantitative cross-sectional line scan analysis as shown in Fig. 4. Because the radius of Al3+ is 0.053 nm,21 much smaller than those of In3+ (0.080 nm) and Zn2+ (0.074 nm),22 Al element are easy to diffuse in the IZO lattices as interstitials or as AlOx clusters. Therefore, Al element in the IZO serve as defects which can reduce the carrier mobility.23 On the other hand, the radius of Zr4+ (0.084 nm) is much larger than that of Al3+,24 and it is more difficult for Zr element to diffuse into the IZO film than for Al element, which can be seen clearly in Fig. 4.
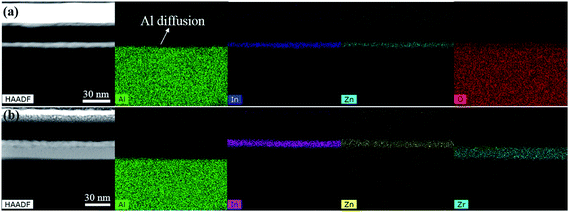 | ||
| Fig. 3 HAADF STEM images together with the elemental distribution detected by EDS for (a) IZO/AlOx region, and (b) IZO/ZrOx/AlOx region. | ||
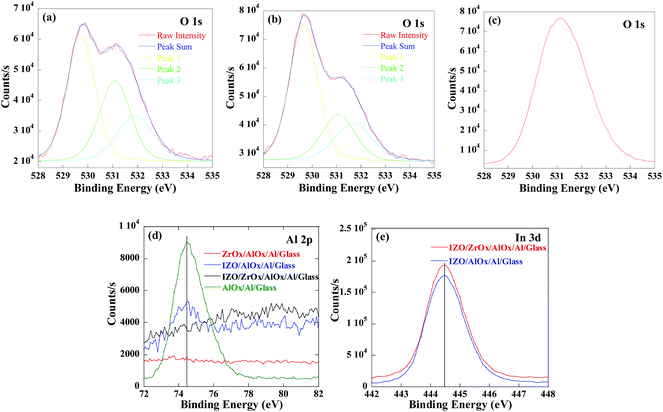
To further investigate the effect of diffusing Al in the IZO films, XPS experiments were performed. Four samples—AlOx/Al/glass, IZO/AlOx/Al/glass, ZrOx/AlOx/Al/glass, and IZO/ZrOx/AlOx/Al/glass—were prepared. Fig. 5(a) and (b) show the O 1s spectra obtained from IZO thin film deposited on AlOx and ZrOx/AlOx, respectively. It was known that the main peak located around 529.7 eV (Peak 1) is related to the oxygen in stoichiometric of the IZO films; the peak at 531.0 eV (Peak 2) is attributed to oxygen vacancies; and the peak located at 532.0 eV (Peak 3) is related to the loosely bound oxygen impurities.25,26 However, it should be noted that the oxygen vacancies herein cannot be characterized by the O 1s spectra, because the peaks attributed to oxygen vacancies and Al–O bond (seen in Fig. 5(c)) are almost at the same position. The higher relative intensity of Peak 2 of the IZO film on bare AlOx compared with that of IZO on ZrOx/AlOx (seen in Fig. 5(a)) was partly ascribed to Al–O bond in the IZO film. Fig. 5(d) and (e) show the Al 2p and In 3d spectra, respectively, for different samples. The Al 2p peak positions for AlOx/Al/glass and IZO/AlOx/Al/glass samples were both at 74.45 eV, which was corresponding to AlOx, supporting that the chemical state of the diffusing Al was the same with that of the anodic AlOx gate insulator. Furthermore, no Al 2p characteristic peak was found in ZrOx/AlOx/Al/glass and IZO/ZrOx/AlOx/Al/glass samples, which indicated the amount of Al element diffusion observed in the Fig. 4(b) could be ignored. In Fig. 5(e), almost no shifts were found for In 3d peaks for the samples with and without ZrOx interlayer, indicating that the chemical states of In were not affected by Al diffusion.22 All these supported that the Al element would only physical diffuse into IZO films on AlOx signal layer as AlOx clusters which acted as impurities in the IZO film, while introduced a ZrOx interlayer remarkably reduce the Al impurities in the IZO films.
Based on these early results and the direct evidence presented here, it can be argued that the enhanced performance obtained in our IZO-TFTs with ZrOx interlayer is attributed to the reduction of the Al diffusion which acts as impurities and the low diffusion effect of the ZrOx itself. Therefore, it can be deduced that introducing an interlayer with low diffusion into the channel layer and having an effect of blocking the elements from gate insulator diffusing into the channel layer is an effective way to improve the performance of the oxide TFTs. To the best of our knowledge, it is for the first time to investigate the insulator–semiconductor diffusion effect of the solution-processed TFTs.
4 Conclusions
In summary, solution-processed IZO-TFTs based on anodized AlOx insulator were fabricated. The IZO-TFTs without ZrOx interlayer showed poor performance with a mobility of only 2.8 cm2 V−1 s−1, a Vth of 4.9 V and a SS of 0.33 V dec−1. By surface modification with the ZrOx interface, the IZO-TFTs exhibited improved performance with a higher mobility of 7.8 cm2 V−1 s−1, a lower Vth of 4.6 V and a lower SS of 0.21 V dec−1. It was supported that the Al element easily diffuse into IZO film and formed AlOx clusters which acted as defects to deteriorate IZO-TFTs electrical properties. By introducing a ZrOx interlayer, the diffusion of Al element was suppressed and the Zr diffusing effect almost could be ignored, both of which reduced the defects in the IZO film and improved the IZO-TFTs performance. Our results suggested that the introduction of an interlayer with less diffusing effect could be an effective way to improve active layer quality and lead to higher electrical performance for solution-processed TFTs.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Acknowledgements
The authors are grateful to the National “863” Project of China (Grant no. 2014AA033002), the National “973” Project of China (Grant no. 2015CB655000), the National Natural Science Foundation of China (Grant nos. 61204087, 51173049, U0634003, 21225418, and 60937001), the Pearl River S&T Nova Program of Guangzhou (Grant no. 2014J2200053), the Guangdong Province Science and Technology Plan (Grant no. 2013B010403004), the Fundamental Research Funds for the Central Universities (Grant no. 2014ZM0003, and 2014ZZ0028), the National Laboratory for Infrared Physics Open Project (M201406), and the Guangdong Innovative Research Team Program (no. 201101C0105067115).References
- K. Nomura, H. Ohta, A. Takagi, T. Kamiya, M. Hirano and H. Hosono, Nature, 2004, 432, 488 CrossRef CAS PubMed.
- E. Fortunato, P. Barquinha and R. Martins, Adv. Mater., 2012, 24, 2945 CrossRef CAS PubMed.
- H. Yabuta, M. Sano, K. Abe, T. Aiba, T. Den, H. Kumomi, K. Nomura, T. Kamiya and H. Hosono, Appl. Phys. Lett., 2006, 89, 112123 CrossRef PubMed.
- J. H. Park, Y. B. Yoo, K. H. Lee, W. S. Jang, J. Y. Oh, S. S. Chae and H. K. Baik, ACS Appl. Mater. Interfaces, 2013, 5, 410 CAS.
- H. Q. Chiang, J. F. Wager, R. L. Hoffman, J. Jeong and D. A. Keszler, Appl. Phys. Lett., 2005, 86, 13503 CrossRef PubMed.
- M. Kim, H. S. Kim, Y. Ha, J. He, M. G. Kanatzidis, A. Facchetti and T. J. Marks, J. Am. Chem. Soc., 2010, 132, 10352 CrossRef CAS PubMed.
- W. Yang, K. Song, Y. Jung, S. Jeong and J. Moon, J. Mater. Chem. C, 2013, 1, 4275 RSC.
- W. Xu, H. Wang, L. Ye and J. Xu, J. Mater. Chem. C, 2014, 2, 5389 RSC.
- J. Ko, J. Kim, S. Y. Park, E. Lee, K. Kim, K. Lim and Y. S. Kim, J. Mater. Chem. C, 2014, 2, 1050 RSC.
- L. Lan and J. Peng, IEEE Trans. Electron Devices, 2011, 58, 1452 CrossRef CAS.
- L. Lan, M. Zhao, N. Xiong, P. Xiao, W. Shi, M. Xu and J. Peng, IEEE Electron Device Lett., 2012, 33, 827 CrossRef CAS.
- P. Xiao, L. Lan, T. Dong, Z. Lin, W. Shi, R. Yao, X. Zhu and J. Peng, Appl. Phys. Lett., 2014, 104, 51607 CrossRef PubMed.
- G. Adamopoulos, S. Thomas, P. H. Wöbkenberg, D. D. C. Bradley, M. A. McLachlan and T. D. Anthopoulos, Adv. Mater., 2011, 23, 1894 CrossRef CAS PubMed.
- B. S. Ong, C. Li, Y. Li, Y. Wu and R. Loutfy, J. Am. Chem. Soc., 2007, 129, 2750 CrossRef CAS PubMed.
- Y. Jung, W. Yang, C. Y. Koo, K. Song and J. Moon, J. Mater. Chem., 2012, 22, 5390 RSC.
- H. Wang, T. Sun, W. Xu, F. Xie, L. Ye, Y. Xiao, Y. Wang, J. Chen and J. Xu, RSC Adv., 2014, 4, 54729 RSC.
- J. Jang, R. Kitsomboonloha, S. L. Swisher, E. S. Park, H. Kang and V. Subramanian, Adv. Mater., 2013, 25, 1042 CrossRef CAS PubMed.
- B. Zhang, H. Li, X. Zhang, Y. Luo, Q. Wang and A. Song, Appl. Phys. Lett., 2015, 106, 93506 CrossRef PubMed.
- J. Yoon, Y. H. Kim, J. Ka, S. Hong, M. H. Yi and K. Jang, J. Mater. Chem. C, 2014, 2, 2191 RSC.
- T. Pan, C. Chen and J. Liu, RSC Adv., 2014, 4, 29300 RSC.
- L. J. Li, H. Deng, L. P. Dai, J. J. Chen, Q. L. Yuan and Y. Li, Mater. Res. Bull., 2008, 43, 1456 CrossRef CAS PubMed.
- D. N. Kim, D. L. Kim, G. H. Kim, S. J. Kim, Y. S. Rim, W. H. Jeong and H. J. Kim, Appl. Phys. Lett., 2010, 97, 192105 CrossRef PubMed.
- C. Lee, Y. Lin and J. Lin, J. Appl. Phys., 2015, 117, 45309 CrossRef PubMed.
- B. M. Reddy, P. Bharali, P. Saikia, A. Khan, S. Loridant, M. Muhler and W. Grunert, J. Phys. Chem. C, 2007, 111, 1878 CAS.
- Y. Jeong, C. Bae, D. Kim, K. Song, K. Woo, H. Shin, G. Cao and J. Moon, ACS Appl. Mater. Interfaces, 2010, 2, 611 CAS.
- G. H. Kim, H. S. Kim, H. S. Shin, B. D. Ahn, K. H. Kim and H. J. Kim, Thin Solid Films, 2009, 517, 4007 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |