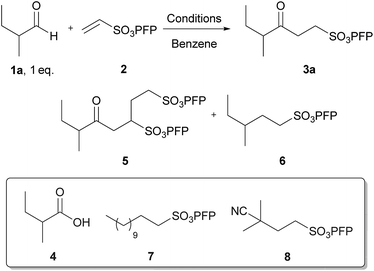
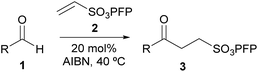
Pentafluorophenyl vinyl sulfonate enables efficient, metal-free, radical-based alkene hydroacylation with an aldehyde as a limiting reagent†
Vijay Chudasama*
Department of Chemistry, University College London, 20 Gordon Street, London, WC1H OAJ, UK. E-mail: v.chudasama@ucl.ac.uk; Tel: +44 (0)20 7679 2077
First published on 12th May 2015
Abstract
The unique properties of pentafluorophenyl vinyl sulfonate allow for a hitherto unmet feat to be realised; efficient and high yielding, metal-free, radical-based alkene hydroacylation employing aldehyde as limiting reagent. The optimised conditions are shown to work in good yields across a series of aldehydes, thus demonstrating the wide applicability of the developed protocol.
Free radical based methodologies offer a complementary approach to the conditions often required for two electron processes, as exemplified in the important role they have played in synthetic organic chemistry.1 The first application of free radical chemistry in the hydroacylation field came in 1949, where Kharasch reported the formation of unsymmetrical ketones from the reaction of aldehydes with alkenes using diacetyl peroxide as a thermally activated initiator.2 Soon after the work by Kharasch, Patrick and Huang reported the benzoyl peroxide-initiated addition of aldehydes to numerous electron deficient alkenes.3 Numerous other examples based on free radical chemistry have been reported since these early articles, with strained double bonds and perfluoroalkenes proving to be particularly good acyl radical acceptors.4 However, an important trend from the early reports to date is the use of the aldehyde component in vast excess, despite the emergence of polarity reversal catalysts.4g,5 Although transition metal-based hydroacylation protocols have achieved hydroacylation using aldehyde as limiting reagent, these protocols come with the disadvantages associated with such procedures (e.g. cost, limited aldehyde scope, toxicity, appreciable decarbonylation).4g,6 Advances in base promoted N-heterocyclic carbene-catalysed intermolecular hydroacylation have also been made, but these remain almost exclusively limited to aromatic aldehydes, due to competing aldol-type chemistry, and highly strained π-acceptors (e.g. cyclopropenes and arynes).7 The lack of a free radical, transition metal-free protocol based on aldehyde functionalisation (i.e. using aldehyde as limiting reagent) may be due to the alkene component not being effective enough in the hydroacylation radical pathway, i.e. an inefficient acyl radical trap and/or a poor chain propagator.
Recently, an aerobically-initiated hydroacylation protocol for the hydroacylation of various electron-poor alkenes (e.g. vinyl sulfonates, vinyl sulfones, unsaturated esters and vinyl phosphonates) has been described.8 In this programme, pentafluorophenyl vinyl sulfonate (PFPVS) was discovered to be exceptionally well-suited to undergo efficient hydroacylation.8f Encouraged by the favourable properties of PFPVS as a radical acceptor/chain carrier, a study to develop a protocol for its use in efficient, metal-free hydroacylation with aldehyde being employed as the limiting reagent was embarked upon (see Fig. 1). Owing to previous work which has shown the products of the hydroacylation of PFPVS, i.e. γ-keto-PFP-sulfonates, to undergo: (i) quantitative elimination to form enones; (ii) elimination–addition to provide an indirect route to the hydroacylation of electron rich alkenes; and (iii) facile conversion to γ-keto-sulfonamides, sultams and sultones,8b,f such a protocol could find application in various fields.8f
The study began by analysing the reaction of PFPVS with a model aldehyde under previously reported aerobically-initiated hydroacylation conditions (Table 1, Entry 1).8b Aware of the well-documented issue of undesirable competing decarbonylation of secondary aldehydes in hydroacylation processes, 2-methyl-butanal 1a was chosen as the model aldehyde to tackle this issue, concurrently, during optimisation. Reaction of an equivalent of 2-methyl-butanal 1a with PFPVS 2 under aerobic activation conditions resulted in a low yield of γ-keto-PFP-sulfonate 3a (Table 1, Entry 1). This was mainly due to the low conversion of alkene, which was a consequence of competing aldehyde auto-oxidation to acid 4. As this competing side-reaction is always likely to exist in any aerobic-based hydroacylation protocol, the use of other, non-aerobic-based initiators was appraised. With the issue of competing decarbonylation being exacerbated with high temperature, the use of low temperature thermal initiators, lauroyl peroxide and azobisisobutyronitrile (AIBN), at 20 mol%, was explored. Initially, reaction of 2-methylbutanal 1a with vinyl sulfonate 2 at 60 °C in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with both initiators was trialled (Table 1, Entries 2 and 3).
1 molar ratio with both initiators was trialled (Table 1, Entries 2 and 3).
| Entry | Temp./°C | Initiatora | 2/eq. | Conversiona 2/% | 3a/% | 5b/% | 6b/% |
|---|---|---|---|---|---|---|---|
| a 20 mol% unless stated otherwise.b Determined by integration of 1H NMR relative to pentachlorobenzene as an internal standard. | |||||||
| 1 | 21 | Air (1 atm) | 1.0 | 58 | 30 | 11 | 0 |
| 2 | 60 | Lauroyl peroxide | 1.0 | 90 | 32 | 8 | 10 |
| 3 | 60 | AIBN | 1.0 | 80 | 46 | 10 | 10 |
| 4 | 40 | AIBN | 1.0 | 70 | 52 | 8 | Trace |
| 5 | 40 | AIBN | 1.2 | 84 | 60 | 11 | Trace |
| 6 | 40 | AIBN | 1.5 | 100 | 64 | 15 | Trace |
| 7 | 40 | AIBN | 2.0 | 100 | 60 | 18 | Trace |
On using lauroyl peroxide, the reaction proceeded with almost complete conversion of vinyl sulfonate, however, only a 32% yield of ketone 3a was obtained. The low yield of ketone and complete conversion of vinyl sulfonate 2 was due to the formation of a significant amount of alkyl sulfonate 7; presumably derived from undecyl radical (generated from thermal decomposition of lauroyl peroxide) addition to vinyl sulfonate 2. In the case of AIBN, reaction at 60 °C with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of 1a
1 molar ratio of 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 resulted in a modest yield of ketone 3a, 46%, with the formation of double addition product 5 and decarbonylated addition product 6 also being observed (Table 1, Entry 3). In contrast to lauroyl peroxide, only a trace quantity of initiator-derived alkyl sulfonate, i.e. compound 8, was observed. This may be a consequence of a less nucleophilic and more sterically crowded tertiary radical resulting from thermal decomposition of AIBN. Alkyl sulfonate 6 is likely to be derived from the secondary radical formed upon decarbonylation of the acyl radical of aldehyde 1a. To reduce the formation of alkyl sulfonate 6, the effect of lowering temperature to suppress decarbonylation was explored. Gratifyingly, lowering the temperature to 40 °C suppressed formation of alkyl sulfonate 6 and increased the yield of ketone 3a to 52% (Table 1, Entry 4). In an attempt to increase conversion of vinyl sulfonate 2, the effect of altering the 1a
2 resulted in a modest yield of ketone 3a, 46%, with the formation of double addition product 5 and decarbonylated addition product 6 also being observed (Table 1, Entry 3). In contrast to lauroyl peroxide, only a trace quantity of initiator-derived alkyl sulfonate, i.e. compound 8, was observed. This may be a consequence of a less nucleophilic and more sterically crowded tertiary radical resulting from thermal decomposition of AIBN. Alkyl sulfonate 6 is likely to be derived from the secondary radical formed upon decarbonylation of the acyl radical of aldehyde 1a. To reduce the formation of alkyl sulfonate 6, the effect of lowering temperature to suppress decarbonylation was explored. Gratifyingly, lowering the temperature to 40 °C suppressed formation of alkyl sulfonate 6 and increased the yield of ketone 3a to 52% (Table 1, Entry 4). In an attempt to increase conversion of vinyl sulfonate 2, the effect of altering the 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio from 1
2 ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was explored (Table 1, Entries 4–7). Despite there being an increase in the formation of double addition product 5, complete conversion was achieved at 1
2 was explored (Table 1, Entries 4–7). Despite there being an increase in the formation of double addition product 5, complete conversion was achieved at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and 1
1.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratios of 1a
2 molar ratios of 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The increased conversion was attributed to more efficient acyl radical trapping by vinyl sulfonate 2 due to its higher concentration. Unsurprisingly, this also resulted in a higher yield of double addition product 5 as the adduct radical that results from acyl radical addition to vinyl sulfonate 2 is also more likely to be trapped by another vinyl sulfonate. Optimal yield, 64%, was achieved at a 1
2. The increased conversion was attributed to more efficient acyl radical trapping by vinyl sulfonate 2 due to its higher concentration. Unsurprisingly, this also resulted in a higher yield of double addition product 5 as the adduct radical that results from acyl radical addition to vinyl sulfonate 2 is also more likely to be trapped by another vinyl sulfonate. Optimal yield, 64%, was achieved at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 molar ratio of 1a
1.5 molar ratio of 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
With optimised conditions in-hand, translation to other electron deficient alkene systems was appraised. Thus the use of ethyl vinyl sulfone, an unsaturated ester and dimethyl vinyl phosphonate as the alkene component was trialled. In contrast to PFPVS 2, trace amounts of hydroacylation products were observed across the series of alkenes (Table 2, Entries 1–3). Even the use of 2,4,6-trichlorophenyl vinyl sulfonate, gave a poor yield of ketone, 21% (Table 2, Entry 4). These results only serve to highlight the unique, favourable properties of PFPVS as an acyl radical acceptor/radical chain carrier.
The aldehyde tolerance of the optimised conditions for the hydroacylation of PFPVS was next explored. A broad range of aldehydes was selected to be appraised under the reaction conditions, aldehydes 1b–p. A mixture of aliphatic and aromatic aldehydes was chosen. The appraisal of aliphatic aldehydes, in particular, was to set the work against a background of aldehydes that give rise to aldol-derived products under harsher ionic-based conditions for hydroacylation. The motivation to examine aromatic aldehydes was due to them being poorly tolerated under previously reported conditions for hydroacylation of PFPVS.8f
Pleasingly, good yields of ketone were generally observed across the broad selection of aldehydes trialled (Table 3). A variety of linear, α-substituted and α,α-disubstituted aldehydes underwent hydroacylation in relatively good yields (Table 3, Entries 1–8). Moreover, the yield observed for isobutyraldehyde 1h was in fact superior to that observed when using previously reported conditions that use a two-fold excess of aldehyde (Table 3, Entry 7).8f Functionalised aldehydes 1j and 1k were also well tolerated under the reaction conditions (Table 3, Entries 9 and 10). As expected, under free radical reaction conditions, application of 10-undecenal 1l resulted in polymerisation and no ketone product was isolated from the reaction mixture (Table 3, Entry 11). Interestingly, however, an internal alkene conjugated with a carbonyl group was better tolerated and afforded ketone in respectable yield (Table 3, Entry 12). Under the mild reaction conditions, minimal to zero aldol-derived product(s) were observed for any of the aliphatic aldehydes. Most pleasingly, use of aromatic aldehydes 1n and 1o also gave the respective ketones in good yield (Table 3, Entries 13 and 14). This is in sharp contrast to what was observed under previously reported reaction conditions, i.e. <10% yield.8f Finally, an aldehyde with an optically enriched α-stereocentre, (S)-2-methylbutanal, was trialled. Reaction proceeded with retention of stereochemistry and highlights another clear advantage over common ionic methods which are more likely to racemise the stereocentre.
| Entry | Aldehyde 1 | Yield 3/% |
|---|---|---|
| a Conditions: aldehyde 1 (1 mmol), vinyl sulfonate 2 (1.5 mmol) and AIBN (20 mol%) in benzene (solvent) under argon at 40 °C. | ||
| 1 | 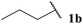 |
71 |
| 2 |  |
68 |
| 3 | 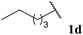 |
72 |
| 4 |  |
61 |
| 5 |  |
60 |
| 6 |  |
70 |
| 7 |  |
62 |
| 8 |  |
51 |
| 9 |  |
61 |
| 10 |  |
64 |
| 11 |  |
0 |
| 12 |  |
54 |
| 13 |  |
64 |
| 14 | 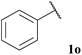 |
61 |
| 15 |  |
62 |
In conclusion, a methodology that allows for a shift from functionalising alkenes to functionalising aldehydes has been presented. This shift in focus has been enabled by the use of PFPVS, owing to its unique properties. The developed protocol has been shown to work across a series of aliphatic, functionalised and aromatic aldehydes in good yields. Moreover, in certain cases, superior yields compared to those obtained in procedures where an excess of aldehyde was employed were observed. The tolerance of an α-enantioenriched aldehyde highlights another advantage of using a mild radical-based protocol. Owing to their established reactivity profile,8b,f the formed γ-keto-PFP-sulfonates will have application in a variety of fields.
Acknowledgements
The author gratefully acknowledges UCL, Dr Richard Fitzmaurice and Professor Stephen Caddick for useful discussions.Notes and references
- (a) W. B. Motherwell and D. Crich, Free Radical Chain Reactions in Organic Synthesis, Academic Press, London, 1992 Search PubMed; (b) G. J. Rowlands, Tetrahedron, 2009, 65, 8603–8655 CrossRef CAS PubMed; (c) G. J. Rowlands, Tetrahedron, 2010, 66, 1593–1636 CrossRef CAS PubMed.
- S. M. Kharasch, W. H. Urry and B. M. Kuderna, J. Org. Chem., 1949, 14, 248–253 CrossRef.
- (a) T. M. Patrick, J. Org. Chem., 1952, 17, 1009–1016 CrossRef CAS; (b) R. L. Huang, J. Chem. Soc., 1956, 1749–1755 RSC.
- (a) L. M. van der Linde and A. J. A. van der Weerdt, Tetrahedron Lett., 1984, 25, 1201–1204 CrossRef CAS; (b) H. Stockman, J. Org. Chem., 1964, 29, 245 CrossRef; (c) R. Dowbenko, J. Am. Chem. Soc., 1964, 86, 946–947 CrossRef CAS; (d) L. Friedman, J. Am. Chem. Soc., 1964, 86, 1885–1886 CrossRef CAS; (e) J. D. LaZerte and R. J. Koshar, J. Am. Chem. Soc., 1955, 77, 910–914 CrossRef CAS; (f) H. Muramatsu and K. Inukai, J. Org. Chem., 1962, 27, 1572–1574 CrossRef CAS; (g) C. Chatgilialoglu, D. Crich, M. Komatsu and I. Ryu, Chem. Rev., 1999, 99, 1991–2069 CrossRef CAS PubMed.
- (a) H. S. Dang and B. P. Roberts, J. Chem. Soc., Perkin Trans. 1, 1998, 67–75 RSC; (b) S. Tsujimoto, T. Iwahama, S. Sakaguchi and Y. Ishii, Chem. Commun., 2001, 2352–2353 RSC; (c) S. Tsujimoto, S. Sakaguchi and Y. Ishii, Tetrahedron Lett., 2003, 44, 5601–5604 CrossRef CAS; (d) L. Melone and C. Punta, Beilstein J. Org. Chem., 2013, 9, 1296–1310 CrossRef CAS PubMed.
- (a) J. C. Leung and M. J. Krische, Chem. Sci., 2012, 3, 2202–2209 RSC and references therein; (b) M. C. Willis, Chem. Rev., 2010, 110, 725–748 CrossRef CAS PubMed; (c) M. C. Willis, Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, ed. G. A. Molander and P. Knochel, Elsevier, Oxford, 2nd edn, 2014, vol. 4, ch. 4.17, pp. 961–994 and references therein Search PubMed.
- (a) F. Liu, X. Bugaut, M. Scheler, R. Fröhlich and F. Glorius, Angew. Chem., Int. Ed., 2011, 50, 12626–12630 CrossRef CAS PubMed; (b) X. Bugaut, F. Liu and F. Glorius, J. Am. Chem. Soc., 2011, 133, 8130–8133 CrossRef CAS PubMed; (c) A. T. Biju and F. Glorius, Angew. Chem., Int. Ed., 2010, 49, 9761–9764 CrossRef CAS PubMed.
- (a) R. J. Fitzmaurice, J. M. Ahern and S. Caddick, Org. Biomol. Chem., 2009, 7, 235–237 RSC; (b) V. Chudasama, R. J. Fitzmaurice, J. M. Ahern and S. Caddick, Chem. Commun., 2010, 46, 133–135 RSC; (c) V. Chudasama, R. J. Fitzmaurice and S. Caddick, Nat. Chem., 2010, 2, 592–596 CrossRef CAS PubMed; (d) V. Chudasama, J. M. Ahern, R. J. Fitzmaurice and S. Caddick, Tetrahedron Lett., 2011, 52, 1067–1069 CrossRef CAS PubMed; (e) V. Chudasama, R. J. Fitzmaurice, D. V. Dhokia, J. M. Ahern and S. Caddick, Chem. Commun., 2011, 47, 3269–3271 RSC; (f) V. Chudasama, A. R. Akhbar, K. A. Bahou, R. J. Fitzmaurice and S. Caddick, Org. Biomol. Chem., 2013, 11, 7301–7317 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra for all compounds. See DOI: 10.1039/c5ra08353b |
| This journal is © The Royal Society of Chemistry 2015 |