Highly porous carbon microflakes derived from catkins for high-performance supercapacitors
Fangwei Ma*,
Jiafeng Wan,
Guang Wu* and
Hui Zhao
Key Laboratory of Chemical Engineering Process & Technology for High-efficiency Conversion, School of Chemistry and Material Science, College of Heilongjiang Province (Heilongjiang University), Harbin 150080, China. E-mail: Fangwei_ma@hotmail.com; wu.guang@163.com; Tel: +86 451 86608616
First published on 12th May 2015
Abstract
Highly porous carbon microflakes (CMFs) with oxygen and nitrogen dual-doping were prepared from willow catkins by pyrolysis at 500 °C in nitrogen, followed by KOH activation and were used as a high-performance supercapacitor electrode material. The as-prepared CMFs with a thickness of 350 nm possess an incredibly high specific surface area of 3510 m2 g−1 and a large pore volume of 2.1 cm3 g−1. The CMFs exhibit a high specific capacitance (for a three-electrode system) of 372, 344 F g−1 at 1 A g−1 in 2 M H2SO4 and 6 M KOH, respectively. The specific capacitance is 278 F g−1 at 1 A g−1 in a two-electrode system (2 M H2SO4 electrolyte). The CMFs electrode demonstrates good rate capability with retention of 79.6% (from 1 A g−1 to 20 A g−1) and excellent cycling stability with 95% capacity retention after 5000 cycles. The CMFs symmetric device shows a high energy density of 9.5 W h kg−1 at a power density of 500 W kg−1 in H2SO4.
Introduction
Supercapacitors, also known as electrochemical capacitors, have attracted significant attention mainly due to their high power density, long lifecycle, and bridging function for the power/energy gap between traditional dielectric capacitors and batteries/fuel cells.1,2 Porous carbons with various microtextures are very attractive and the most widely used electrode materials for supercapacitors due to their large surface area, relatively good electrical properties and moderate cost.3,4 Especially, porous carbon derived from biomass has attracted a great degree of interest as a promising electrode material since it is abundant, environmentally friendly and low cost.5,6 Presently, the biomass wastes are just burnt producing ash and hazardous gaseous pollution products. If the use of natural renewable resources and low-cost biomass wastes converting into high value-added porous carbon materials, it is a very meaningful and valuable research work.Up to now, many biomasses, such as straw,7 pistachio shell,8 banana fibers,9 sugar-cane bagasse,10,11 and kenaf stemm,12 were successfully converted into porous carbons and used as electrode materials for supercapacitors. The simplest approach to produce porous materials from biomass waste materials is pyrolysis of them under an inert atmosphere and the further chemical or physical activation to develop the porosity and enhance surface area.13 For instance, porous carbon derived from celtuce leaves with ultra-high specific surface area of 3404 m2 g−1 and the specific capacitance of 273 F g−1 at current densities of 0.5 A g−1 in two-electrode systems.14 Functional microporous carbon from plant leaves has exhibited a very high specific capacitance of 302 F g−1 at 1 A g−1 in H2SO4 electrolyte.15 Recently, hierarchically porous carbons with 1D to 3D network are attracting vast interest for high-performance electrode materials.16,17 The 3D nanoscaled architecture can not only provide a continuous electron pathway to ensure good electrical contact, but also facilitate ion transport by shortening diffusion pathways. 1D carbon microfibers prepared from cocoon microfibers exhibited superior performance for supercapacitor electrode.18 3D porous carbon aerogel derived from bagasse has been recently reported as high performance supercapacitor electrode,19 demonstrating the possibility for the large scale production of hierarchical porous carbons by choosing low-cost biomass waste. Very recently, 2D flake-like porous carbon, as excellent supercapacitor electrode materials, has also been obtained from biomass wastes. Jin et al. reported microporous carbon nanoplates from regenerated silk proteins. The carbonized material with high surface areas and morphological characteristics advantageous exhibited a specific capacitance of 264 F g−1 at 0.1 A g−1 in 1 M H2SO4 electrolyte.20 Yan et al. just reported porous carbon flakes derived from human hair with a high specific capacitance of 340 F g−1 in 6 M KOH at 1 A g−1 and good stability over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.21 So, it is very essential to prepare 3D hierarchical carbon materials with excellent performance by exploring precursors such as biomass, which is low-cost, readily available and renewable.
000 cycles.21 So, it is very essential to prepare 3D hierarchical carbon materials with excellent performance by exploring precursors such as biomass, which is low-cost, readily available and renewable.
Catkins, as seeds of willows, are readily available biomass waste generated from willows every spring. Willow catkins have a hollow, tube-like structure, from which carbon microtubes have been prepared.22 To our knowledge, there is no report on activated carbon prepared from catkins for supercapacitor. In this study, we demonstrate the preparation of 2D carbon microflakes (CMFs) with ultra-high surface area via carbonization and KOH activation of catkins. The CMFs show an extremely high specific surface area of up to 3510 m2 g−1 and exhibit remarkable performance as supercapacitor electrode materials. The high specific capacitance is up to 372, 278 F g−1 at a current density of 1 A g−1 with 2 M H2SO4 electrolyte in three and two-electrode systems, respectively.
Experimental
Preparation of porous CMFs
The willow catkins were collected in the campus of Heilongjiang University, China. The catkins were washed using deionized water and dried at 80 °C, and then pyrolyzed at 500 °C for 1 h under a nitrogen atmosphere. The pre-carbonized catkins were mixed with KOH based on mass ratio, and the mixtures were heated in a quartz tube at 800 °C for 1 h with a heating rate of 5 °C min−1 under a nitrogen atmosphere. After cooling down to room temperature, the black sample was washed with 1 M HCl solution and then thoroughly washed with deionized water. The carbon materials were collected by centrifugation and dried at 120 °C overnight. The resultant catkins derived porous carbon microflakes (CMFs) are denoted as CMF-1 and CMF-2, where 1, 2 indicate the mass ratio of KOH with pre-carbonized catkins (0.15–0.2 g CMFs can be roughly obtained from 1 g catkins after carbonization and activation).Characterization
Scanning electron microscopy (SEM) micrographs were acquired using a Hitachi S-4800 instrument. Transmission electron microscopy (TEM) was performed with a JEM-2100 electron microscope (JEOL, Japan). XRD analysis was conducted on Rigaku D/max-IIIB with copper radiation (CuKα, 0.15406 nm). Raman spectra were performed on a Jobin Yvon HR 800 micro-Raman spectrometer at 457.9 nm. The XPS measurements were performed on a VG ESCALAB MK II (VGScientific, UK). The nitrogen adsorption and desorption isotherms were measured at −196 °C with a Quadrachrome AUTOSORB-1-MP Adsorption Instrument.Electrochemical measurements
All the electrochemical measurements were carried out on a CHI 660E electrochemical workstation (Shanghai Chenhua, China) in 2 M H2SO4 solution. To prepare the testing electrode, CMFs were mixed with acetylene black and poly(tetrafluoroethylene) at a mass ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, pressed onto titanium mesh (the active mass is about 3.0 mg). For a three-electrode system, the above prepared electrode, platinum foil (2.5 × 2.5 cm2) and Ag/AgCl were used as working electrode, counter electrode and reference electrode, respectively. Impedance measurements were conducted over a frequency range from 100 kHz to 10 mHz at open circuit potential with the amplitude of 5 mV. The specific gravimetric capacitance was calculated from the discharge process according to:
10, pressed onto titanium mesh (the active mass is about 3.0 mg). For a three-electrode system, the above prepared electrode, platinum foil (2.5 × 2.5 cm2) and Ag/AgCl were used as working electrode, counter electrode and reference electrode, respectively. Impedance measurements were conducted over a frequency range from 100 kHz to 10 mHz at open circuit potential with the amplitude of 5 mV. The specific gravimetric capacitance was calculated from the discharge process according to:where I (A) is the charge–discharge current, m (g) is the mass of active material, and ΔV (V) is the voltage change within the discharge time Δt (s) excluding the IR drop during the discharge process.
For a two-electrode system, the two symmetrical electrodes and a porous polypropylene separator were sandwiched together in a poly(tetrafluoroethylene) cell for a two-electrode system. The specific capacitance for the single electrode was calculated according to:
where Et (W h kg−1) is the specific energy density, Pt (W kg−1) is the specific power density, Ct (F g−1) is the specific capacitance of the total symmetrical system, ΔV (V) is the cell voltage for charging and discharging excluding the IR drop during the discharge process, and Δt (s) is the discharge time.
Results and discussion
Scheme 1 illustrates a schematic diagram for the preparation of porous carbon from willow catkins. The cleaned and dried catkins were pre-carbonized at 500 °C. The pre-carbonized catkins were mixed at different weight ratios of KOH and activated at 800 °C for 60 min.Fig. 1a shows the micrograph of pre-carbonized catkins. Partly broken carbon microtubes were obtained, the diameter of which is about 8 μm. The carbon microflakes were easily obtained from these microtube walls by grinding. The SEM and TEM images (Fig. 1b and c) show the morphology of carbon microflakes, the thickness of which is about 350 nm (inset in Fig. 1b). High-resolution transmission electron microscope (HRTEM) images are used to identify the microstructure of these carbon microflakes, the result (Fig. 1d) clearly illustrates that porous carbon flakes with a uniform, large disorder texture and meso/micropores channels can be produced by activation of pre-carbonized catkins. There are some short fringes with a few nanometers long more clearly observed near the edges of the samples, representing graphene-type layers, highly misoriented, some of them forming stacks of a few layers. The disorientation of the graphene-type units causes the porosity of the material.
 | ||
| Fig. 1 SEM images of pre-carbonized catkins (a); SEM image (b), TEM image (c) and (d) HRTEM image of CMF-2. | ||
Fig. 2a shows powder X-ray diffraction (XRD) in the wide-angle region of these CMFs materials. There are two diffraction peaks centered at 24.1° and 43.7°, which can be indexed to the (002) and (101) diffraction from graphitic phase.23 The diffraction peaks are relatively low in intensity and broad in shape, which are often related to the turbostratic carbon structure having disoriented graphene-type units. It also can be seen that the peaks of CMF-2 is not as visible as that of CMF-1, indicating the more amorphous structure of CMF-2 resulting from the further harsh KOH-activation. The Raman spectra (Fig. 2b) for the CMF samples exhibits two peaks centered at 1362 cm−1 (D band) and 1594 cm−1 (G band),24 which are related to disordered graphite carbon and graphitic carbon phase, respectively. The intensity ratio of D/G bands (ID/IG) indicates the degree of structural order with respect to a perfect graphitic structure. The ID/IG values are determined to be 0.81 and 0.83 for CMF-1 and CMF-2, respectively, reflecting their lower graphitic order degree. These results from HRTEM image, XRD pattern and Raman spectra demonstrate that the catkins-derived CMFs are typical of activated carbon materials with an amorphous-like structure.
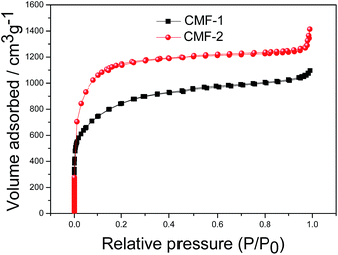
The porosity of the CMFs was investigated by nitrogen adsorption–desorption experiment, the isotherms are presented in Fig. 3. The adsorption–desorption isotherms of CMFs are all of type I for microporous materials (shown in Fig. 3). The volume of nitrogen adsorbed at a relative pressure (P/P0) of ∼1 increases with enhancing the dosage of KOH, indicating the increased number or accessibility of small pores. The porous properties of CMFs are summarized in Table 1, it can be seen that the highly porous CMFs with ultra-high specific surface area were obtained by activation of pre-carbonized catkins. It is found that KOH activation plays an important role in the development of the pore structure. With increasing the dosage of KOH (the weight ratio of KOH and carbon from 1 to 2), the BET surface area and total pore volume of CMFs increase from 2857 to 3510 m2 g−1 and 1.7 to 2.1 cm3 g−1, respectively. These values are all much higher than that of activated carbons derived from pomelo peel reported recently,25 which are only 2191 m2 g−1 and 1.034 cm3 g−1. High surface area and ultrahigh total pore volume are significantly desired for enhanced performance of hierarchically porous carbon materials. Hence, we propose that the CMFs will exhibit satisfying supercapacitor performance.
| Sample | SBET (m2 g−1) | Vpore (cm3 g−1) | Daver (nm) | Cs (F g−1) |
|---|---|---|---|---|
| CMF-1 | 2857 | 1.7 | 2.38 | 296 |
| CMF-2 | 3510 | 2.1 | 2.43 | 372 |
The surface chemical composition of CMFs samples were characterized by XPS and the results are given in Table 2. The chemical compositions of these CMFs materials were found to consist of C, O, N, S and P. The amounts of oxygen and nitrogen are 9.93% and 2.82% in CMF-1 and 7.57% and 0.82% in CMF-2, respectively. The contents of these heteroatoms (O, N, S and P) are inversely proportional to the weight of KOH. The oxygen heteroatom content is relatively high by comparing with other heteroatoms. To evaluate the chemical identities of the heteroatoms O and N in the CMFs, the deconvolution of the N1s and O1s spectrums are presented in Fig. 4. The O1s peaks at 531.6 and 533.0 eV are assigned to oxygen in carbonyl or quinone groups (O-1), oxygen in esters and in hydroxyls (O-2), respectively.26 For nitrogen, the N1s spectrum can be fitted by three peaks located at 398, 400 and 401 eV, which are attributed to pyridinic-N (N-6), pyrrolic/pyridine-N (N-5) and quaternary (N-Q), respectively.27 O-1, O-2, N-6 and N-5 would exert pronounced influence on the electrochemical capacitance properties of carbon related materials, due to their desirable pseudo-capacitive performance and improved wettability.28,29 By fitting the N1s and O1s core level spectra for the two samples, it reveals that the relative surface content of each nitrogen and oxygen species has remained little changed with varying the consumption of KOH activator.
| Sample | C1s (at%) | O1s (at%) | N1s (at%) | S2p (at%) | P2p (at%) |
|---|---|---|---|---|---|
| CMF-1 | 86.92 | 9.93 | 2.82 | 0.12 | 0.22 |
| CMF-2 | 91.41 | 7.57 | 0.82 | 0.03 | 0.18 |
Based on the above discussions, it is expected that CMFs may exhibit impressive electrochemical performance for supercapacitor, due to high surface area, 2D microflake structure and presence of heteroatoms. The performance of CMFs materials as supercapacitor electrodes were first measured by using a three-electrode system in H2SO4 aqueous solution. Fig. 5a shows the CV curves of CMF-1 and CMF-2 at a scan rate of 10 mV s−1, all the CV curves present rectangular-like shape with the appearance of reversible humps at ∼0.5 V, indicating their typical double layer capacitive behavior and a large pseudo-capacitance linked to the heteroatom functionalities. Compared to the CMF-1, the CMF-2 exhibits a much larger CV loop, suggesting the increase of capacitance with enhancing the weight of KOH activator. CV curves of CMF-2 at scan rates between 5 and 100 mV s−1 are shown in Fig. 5b. The quasi-rectangular shape of the CV curves changed slightly with the enhancement of scan rate, and the appearance of roughly rectangular-like shapes remained even at high scan rate (100 mV s−1), indicating it has good rate performance. Fig. 5c presents the galvanostatic charge–discharge curves of CMF materials at a current density of 1 A g−1. The slight deviation from a linear shape to the extent at ∼0.5 V confirms the existence of pseudocapacitance coming from doped heteroatoms.30 The galvanostatic charge–discharge curves of CMF-2 at various current densities (from 1 to 20 A g−1) are also shown in Fig. 5d. It can be also found that the IR drop (i.e. potential drop) at the start of a discharge cycle is 8.8 mV at a current density of 1 A g−1, and the IR drop increases with enhancing current density (40 mV at 5 A g−1). The relatively higher IR drop results mainly from the electrical connection resistance (poor conductivity of titanium mesh) and resistance of ion migration in carbon micropores. The specific gravimetric capacitances were calculated from the discharge process at different current densities ranged from 1 to 20 A g−1. At 1 A g−1, the specific capacitance of 296 and 372 F g−1 were obtained for CMF-1, CMF-2, respectively. The capacitance value of CMF-2 is higher than the case of reported recently human hair-derived carbon flakes (340 F g−1 at a current density of 1 A g−1).21
Rate capability is also an important feature for supercapacitor. The rate performance of CMFs is further evaluated by the relationships between specific capacitance and charge–discharge current density (Fig. 5e). The specific capacitance decreased gradually with the increase of current density, possibly due to low electronic conductivity and inadequate time for electrolyte diffusion into the inner micropores at high charge current densities. The specific capacitance of CMF-2 is higher than that of CMF-1 at all tested current densities, because of its much higher surface area. Surprisingly, even at a high current density of 10 and 20 A g−1, the high specific capacitance could maintained as high as 265 and 246 F g−1, respectively. This performance is superior to porous carbon derived from the shell of broad beans (202 F g−1 at 0.5 A g−1),31 the honeycomb-like porous carbon (212 F g−1 at 20 A g−1)32 and activated carbons derived from pomelo peel (250 F g−1 at 10 A g−1).25 The superior capacitance performance of CMFs could be attributed to the synergistic effect of high surface area with large number of tiny micropores for charge storage, unique microflake structure contributing to short diffusion path during the charge–discharge processes and doped heteroatoms (O, N) improving its wettability.33
To credibly determine the electrochemical performance of CMF-2, further measurements were also conducted in a two-electrode system with the H2SO4 electrolyte. The galvanostatic charge–discharge curves from 1 to 4 A g−1 (Fig. 6a) display slightly nonlinear shapes, indicating the presence of redox reactions during the charge–discharge process. Based on the charge–discharge curves, the gravimetric capacitance for the single electrode is 278 F g−1 at 1 A g−1, demonstrating its better performance than the carbon derived from celtuce leaves (the specific capacitance is 273 F g−1 at 0.5 A g−1).14 The capacitances for a single electrode at current densities ranged from 1 to 10 A g−1 are shown in Fig. 6b. The capacitance is 256, 225 and 200 F g−1 at current densities of 2, 4 and 10 A g−1, respectively. When the current density increases by a factor of 10 (from 1 A g−1 to 10 A g−1), the capacitance retention of the CMF-2 is up to 72%. The capacitances are apparently higher than that of the bean dregs derived carbon materials (210 F g−1 at 1 A g−1),34 porous carbon aerogel derived from bagasse (142 F g−1 at 0.5 A g−1)19 and the 3D hierarchical porous carbon (236 F g−1 at 2 A g−1).35 The Ragone plot for the CMF-2 symmetrical system (Fig. 6c) shows that the energy density is about 9.5 W h kg−1 at power density of 500 W kg−1, which is superior to honeycomb-like porous carbon derived from pomelo peel (a maximum energy density of 9.4 W h kg−1 at a powder density of 96 W kg−1).32 The energy density is 5.7 W h kg−1 at high power density of approximately 4500 W kg−1. The long-term cyclic stability of the CMF-2 electrode was investigated using galvanostatic charge–discharge measurement at the high current density of 5 A g−1 in the two-electrode system, and the results are presented in Fig. 6d. After 5000 cycles, the capacities still maintain at 95% of the initial specific capacitance, which is superior to the nitrogen doped porous carbon synthesized from yogurt (91% after 5000 charge–discharge cycles).36 This result displays the remarkable cycling performance of CMFs.
The CV and galvanostatic charge–discharge of the CMF-2 were also measured in three-electrode configuration with 6 M KOH solution as electrolyte. The CV curves at different scan rates 10 to 200 mV s−1 are shown in Fig. 7a. The CV curves display a rectangular-like shape with obvious hump at low potential region even at high scan rate of 200 mV s−1, indicating a dominant electric double-layer capacitance behavior with reversible pseudocapacitance from oxygen and nitrogen groups. The galvanostatic charge–discharge curves exhibited in Fig. 7b show the approximately triangular symmetrical distributions and show a small voltage drop implying a low equivalent series resistance. The specific capacitances at different current densities (from 1 to 20 A g−1) are given in Fig. 7c. The specific capacitance is 344 F g−1 at 1 A g−1, which is higher than that of hierarchical nanoporous carbon derived from bamboo-based industrial byproduct (301 F g−1 at 0.1 A g−1)37 and biomass-based activated carbons (279 F g−1 at 1 A g−1).38 Even at a higher current density of 20 A g−1, the specific capacitance of 274 F g−1 is still retained, which is superior to porous carbon from fermented rice (219 F g−1 at 15 A g−1).39 The CMF-2 has superior rate capability with retention of 79.6% (from 1 A g−1 to 20 A g−1), which is better than that in H2SO4 electrolyte (66%).
Conclusions
In summary, porous carbon microflakes with ultra-high specific surface area were first prepared from willow catkins though pre-carbonization and KOH activation. The thickness of carbon microflakes is about 350 nm. The as-prepared CMFs possess extremely high specific surface area of 3510 m2 g−1 and are enriched oxygen heteroatoms. The CMFs have exhibited high charge storage capacity with a specific capacitance of 372 F g−1 at 1 A g−1 with 2 M H2SO4 electrolyte and 344 F g−1 at 1 A g−1 with 6 M KOH electrolyte in a three-electrode system. The CMFs also shows high specific capacitance of 278 F g−1 at 1 A g−1 in a two-electrode system and excellent cycle performance (95% capacity retention after 5000 cycles). This good supercapacitor performance can be attributed to high surface area, unique microflake morphology and doped heteroatoms. These excellent results demonstrate the applicability of catkins derived porous carbon microflakes materials for high-performance supercapacitors.Acknowledgements
This work was supported by national Natural Science Foundation of China (21406056) and Science & Technology Research Foundation of Heilongjiang Province Education Bureau of China (no. 12541626).Notes and references
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245 CrossRef CAS.
- Q. Zhang, E. Uchaker, S. L. Candelariaz and G. Cao, Chem. Soc. Rev., 2013, 42, 3127 RSC.
- L. L. Zhang, Y. Gu and X. S. Zhao, J. Mater. Chem. A, 2013, 1, 9395 CAS.
- F. Béguin, V. Presser, A. Balducci and E. Frackowiak, Adv. Mater., 2014, 26, 2219 CrossRef PubMed.
- S. Dutta, A. Bhaumik and K. C.-W. Wu, Energy Environ. Sci., 2014, 7, 3574 CAS.
- M. Sevilla and R. Mokaya, Energy Environ. Sci., 2014, 7, 1250 CAS.
- L. Wei, M. Sevilla, A. B. Fuertes, R. Mokaya and G. Yushin, Adv. Energy Mater., 2011, 1, 356 CrossRef CAS PubMed.
- F. Wu, R. Tseng, C. Hu and C. Wang, J. Power Sources, 2005, 144, 302 CrossRef CAS PubMed.
- V. Subramanian, C. Luo, A. M. Stephan, K. S. Nahm, S. Thomas and B. Wei, J. Phys. Chem. C, 2007, 111, 7527 CAS.
- T. E. Rufford, D. Hulicova-Jurcakova, K. Khosla, Z. Zhu and G. Q. Lu, J. Power Sources, 2010, 195, 912 CrossRef CAS PubMed.
- M. Wahid, D. Puthusseri, D. Phase and S. Ogale, Energy Fuels, 2014, 28, 4233 CrossRef CAS.
- L. Wang, Y. Zheng, Q. Zhang, L. Zuo, S. Chen, S. Chen, H. Hou and Y. Song, RSC Adv., 2014, 4, 51072 RSC.
- J. Wang and Q. Liu, RSC Adv., 2015, 5, 4396 RSC.
- R. Wang, P. Wang, X. Yan, J. Lang, C. Peng and Q. Xue, ACS Appl. Mater. Interfaces, 2012, 4, 5800 CAS.
- M. Biswal, A. Banerjee, M. Deo and S. Ogale, Energy Environ. Sci., 2013, 6, 1249 CAS.
- Z. Yu, L. Tetard, L. Zhai and J. Thomas, Energy Environ. Sci., 2015, 8, 702 CAS.
- X. He, H. Zhang, H. Zhang, X. Li, N. Xiao and J. Qiu, J. Mater. Chem. A, 2014, 2, 19633 CAS.
- Y. Liang, D. Wu and R. Fu, Sci. Rep., 2013, 3, 1119 Search PubMed.
- P. Hao, Z. Zhao, J. Tian, H. Li, Y. Sang, G. Yu, H. Cai, H. Liu, C. P. Wong and A. Umar, Nanoscale, 2014, 6, 12120 RSC.
- Y. S. Yun, S. Y. Cho, J. Shim, B. H. Kim, S. J. Chang, S. J. Baek, Y. S. Huh, Y. Tak, Y. W. Park, S. Park and H. J. Jin, Adv. Mater., 2013, 25, 1993 CrossRef CAS PubMed.
- W. Qian, F. Sun, Y. Xu, L. Qiu, C. Liu, S. Wang and F. Yan, Energy Environ. Sci., 2014, 7, 379 CAS.
- Y. Ma, J. Zhao, L. Zhang, Y. Zhao, Q. Fan, X. Li, Z. Hu and W. Huang, Carbon, 2011, 49, 5292 CrossRef CAS PubMed.
- M. Li, C. Liu, H. Cao, H. Zhao, Y. Zhang and Z. Fan, J. Mater. Chem. A, 2014, 2, 14844 CAS.
- M. Zhou, F. Pu, Z. Wang and S. Guan, Carbon, 2014, 68, 185 CrossRef CAS PubMed.
- C. Peng, J. Lang, S. Xu and X. Wang, RSC Adv., 2014, 4, 54662 RSC.
- Q. Li and A. D. Lueking, J. Phys. Chem. C, 2011, 115, 4273 CAS.
- F. W. Ma, H. Zhao, L. P. Sun, Q. Li, L. H. Huo, T. Xia, S. Gao, G. S. Pang, Z. Shi and S. H. Feng, J. Mater. Chem., 2012, 22, 13464 RSC.
- L. Hao, X. Li and L. Zhi, Adv. Mater., 2013, 25, 3899 CrossRef CAS PubMed.
- G. Zheng, L. Hu, H. Wu, X. Xie and Y. Cui, Energy Environ. Sci., 2011, 4, 3368 CAS.
- L. Zhao, L. Z. Fan, M. Q. Zhou, H. Guan, S. Qiao, M. Antonietti and M. M. Titirici, Adv. Mater., 2010, 22, 5202 CrossRef CAS PubMed.
- G. Xu, J. Han, B. Ding, P. Nie, J. Pan, H. Dou, H. Li and X. Zhang, Green Chem., 2015, 17, 1668 RSC.
- Q. Liang, L. Ye, Z.-H. Huang, Q. Xu, Y. Bai, F. Kang and Q.-H. Yang, Nanoscale, 2014, 6, 13831 RSC.
- H. Sun, L. Cao and L. Lu, Energy Environ. Sci., 2012, 5, 6206 CAS.
- C. Ruan, K. Ai and L. Lu, RSC Adv., 2014, 4, 30887 RSC.
- L. Qie, W. Chen, H. Xu, X. Xiong, Y. Jiang, F. Zou, X. Hu, Y. Xin, Z. Zhang and Y. Huang, Energy Environ. Sci., 2013, 6, 2497 Search PubMed.
- M. Wahid, G. Parte, D. Phase and S. Ogale, J. Mater. Chem. A, 2015, 3, 1208 CAS.
- W. Tian, Q. Gao, Y. Tan, K. Yang, L. Zhu, C. Yang and H. Zhang, J. Mater. Chem. A, 2015, 3, 5656 CAS.
- K. Wang, N. Zhao, S. Lei, R. Yan, X. Tian, J. Wang, Y. Song, D. Xu, Q. Guo and L. Liu, Electrochim. Acta, 2015, 166, 1 CrossRef CAS PubMed.
- S. Gao, Y. Chen, H. Fan, X. Wei, C. Hu, H. Luo and L. Qu, J. Mater. Chem. A, 2014, 2, 3317 CAS.
| This journal is © The Royal Society of Chemistry 2015 |