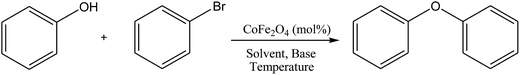
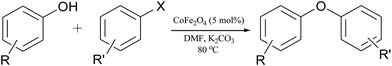
Application of nickel ferrite and cobalt ferrite magnetic nanoparticles in C–O bond formation: a comparative study between their catalytic activities†
Firouz Matloubi Moghaddam*,
Ghazal Tavakoli and
Ali Aliabadi
Laboratory of Organic Synthesis and Natural Products, Department of Chemistry, Sharif University of Technology, Azadi Street, PO Box 111559516, Tehran, Iran. E-mail: matloubi@sharif.edu
First published on 1st July 2015
Abstract
Magnetic nanoparticles of nickel ferrite and cobalt ferrite were synthesized and their catalytic activity in the C–O bond formation reaction was compared. Both these spinel systems can catalyze the reaction between various types of aryl halides and phenol derivatives efficiently. The catalysts are easily removed from the reaction medium and can be reused further in several runs. All the reactions resulted in very good to excellent yields under optimized reaction conditions; however, reactions using nickel ferrite as catalyst were completed in relatively shorter reaction times. Also, analyses showed that the reusability of the two catalytic systems is approximately comparable.
Introduction
The C–O coupling reaction generating diaryl ether derivatives has received a lot of attention in recent years.1–7 These aryl ethers are not only an important class of ‘building blocks’ that are widely employed in organic synthesis, polymer industry, pharmaceutics and agrochemical areas, but can also be found in many synthetically challenging and medicinally important natural products.8–13 These moieties are extremely versatile intermediates of medicinally active compounds such as ancomycin teicoplanin (antimicrobial),14 piperazinomycin (antifungal), K-13 (antiviral peptide), bouvardin (antitumoral) and many others.15On the other hand, diaryl ethers are traditionally synthesized via nucleophilic aromatic substitution or transition metal-catalyzed Ullmann type reaction.16 The later has suffered from some main drawbacks such as harsh reaction conditions (high temperatures and long reaction times) and stoichiometric use of the copper reagent.1a,9,10,16a,17 Laterally, to overcome these issues, many various transition metal-based catalytic systems were used in this reaction to allow the diaryl ether be obtained under milder conditions. Among them, application of palladium and copper in the presence of different ligands has been most reported.1–4,18 However, use of palladium catalysts has their own disadvantages including contamination to products, high cost, toxicity, moisture-sensitive nature, hard recovery and the non-commercial sophisticated phosphine ligands.19 Therefore, research for finding new catalytic systems enabling the formation of C–O bonds between aryl halides and alcohols having none of the aforementioned drawbacks has been continued and many advances has gained up to now.
Recently, nanoparticles have been widely used in many different areas in organic chemistry.20 One of these applications is as catalyst. In fact, nanocatalysts due to their large surface area show very excellent catalytic activity. A new class of materials for catalysis is spinel bimetallic nanoparticles, a category of nanomaterials having new physical and chemical properties stemmed from synergistic effects between the two metals;20–23 so, they are highly desirable for specific technological applications, especially as catalyst. In these systems, not only the nature of the transition metals employed for preparation of the catalyst, but also the size and morphology of the particles can affect the catalytic activity of the system.20 Nickel ferrite and cobalt ferrite nanoparticles, denoted as NiFe2O4 and CoFe2O4 respectively, are of the most important members of oxide-based spinel ferrites.22,23 In this structure, ferric ions are located at tetrahedral sites and nickel/cobalt ions at octahedral sites having anti parallel spines leading to ferromagnetism.23 Furthermore, it is demonstrated that when the diameter of particle is less than that of a single magnetic domain, spinel ferrite nanoparticles become superparamagnetic, which is very important in macroscopic quantum tunneling between spin states.24 So, due to this ferromagnetic property, these nanoparticles can be categorized as recoverable and reusable catalysts; because of their convenient separation from the reaction media using an external magnetic field. On the other hand, rapid electron exchange between tetrahedral and octahedral sites enhances their catalytic activity.25 These compounds display very good catalytic activity in various organic reactions.22,23 The main advantages reported for cobalt ferrite nanoparticles are their good mechanical and excellent chemical stabilities at higher temperature, strong anisotropy, moderate saturation magnetization and high coercivity.25
Previously, we tested nickel ferrite nanoparticles in Sonogashira, amination and cyanation reaction of aryl halides. The reactions were carried out under conventional heating and resulted in excellent yields. Herein, in continuation of our previous works,26–28 we wish to report the application of two highly active recyclable spinel catalysts, i.e. cobalt ferrite (1) and nickel ferrite (2) for C–O coupling reaction of different kinds of aryl halides under conventional heating conditions. In comparison with previous unrecyclable catalysts based on other transition metals,1–4,7 these catalytic systems not only are low-cost, but also are readily prepared in few steps from available starting materials. Another main advantage of these catalysts is their complete and easy removal from the reaction media after the completion of the reaction due to its ferromagnetic nature that make it possible to reuse it further in consecutive runs. This is very important because the remaining metal-based catalyst in the product can be harmful; especially in the cases that the end product is directly associated with human life such as in pharmaceutical synthesis. Because of the nanosize characteristic of these catalysts that provides a large accessible surface area for better interaction with reactants in the media, all reactions can be performed efficiently using small amount of the catalysts in short times compared to previous reports.29 In 2012, Xu et al. reported an Ullmann type C–O coupling reaction using magnetic copper ferrite nanoparticles.30 In their work, they employed 5 mol% of the spinel catalysts in the presence of 10 mol% of a diketone ligand, i.e. 2,2,6,6-tetramethylheptane-3,5-dione and cesium carbonate as base at 135 °C (Scheme 1a). Next in 2013, Qian et al. reported a copper-catalyzed Ullmann-type synthesis of diaryl ethers in the presence of aminophenols as ligand.31 Formation of diaryl ethers in their work were performed using Cu(I) in acetonitrile during 24 h. In addition, only liable aryl halides, i.e. aryl iodides and bromides could successfully be employed (Scheme 1b). Application of CuO nanoparticles as catalyst in C–O coupling reaction in the presence of KF/Clinoptilolite as a solid base was described by Khalilzadeh and his co-workers in 2014.32 Again, the reaction is restricted to aryl iodides and bromides and is carried out under harsh reaction conditions (at 120 °C using DMSO as solvent under nitrogen atmosphere and during 18–30 h) (Scheme 1c). Herein, using our catalytic systems, we can improve the reaction conditions and accelerate the formation of C–O bonds between aryl halides and phenol derivatives under milder and ligand-free conditions (Scheme 1d). The catalytic activities of these catalysts were comparable; however, nickel ferrite nanoparticles could catalyze the reactions in approximately shorter reaction times and with comparable to higher yields than cobalt ferrite ones.
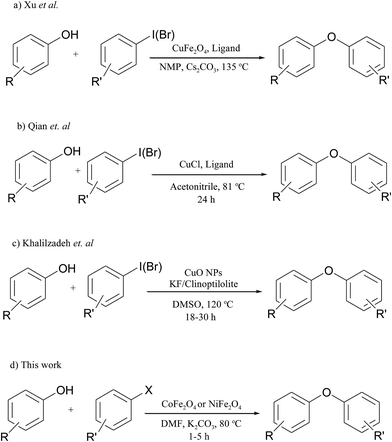 | ||
| Scheme 1 C–O bond formation reaction using various types of catalytic systems under different conditions. | ||
Result and discussion
NiFe2O4 and CoFe2O4 nanoparticles were prepared using co-precipitation method according to our previous reported procedure and Zhang et al., respectively.27,33 Structures of both catalysts were characterized by analyzing their XRF results, XRD patterns and FTIR spectra. Also, TEM and SEM images of both samples were obtained along with their magnetization curves.In the next step, after optimizing reaction conditions, these nanoparticles were employed as catalyst in the C–O cross coupling reaction between aromatic alcohols and various types of aryl halides. The catalytic activities of each of these catalysts were explored in the same conditions and their results were compared.
Initially, in order to find the optimized reaction conditions, the C–O cross-coupling reaction of bromobenzene and phenol was selected as the model reaction. Various types of bases and solvents at different temperatures and in the presence of various concentrations of cobalt ferrite nanoparticles as catalyst were employed in this reaction and their results were investigated to find the best reaction conditions. The results are shown in Table 1.
| Entry | Solvent | Base | Catalyst (mol%) | Temperature (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction condition: bromobenzene (1 mmol), phenol (1 mmol), base (1.2 mmol), cobalt ferrite nanoparticles, solvent and temperature.b Isolated yield. | ||||||
| 1 | DMF | K2CO3 | 10 | 100 | 50 | 91 |
| 2 | Toluene | K2CO3 | 10 | 100 | 50 | 59 |
| 3 | H2O | K2CO3 | 10 | Reflux | 50 | 38 |
| 4 | EtOH | K2CO3 | 10 | Reflux | 50 | 44 |
| 5 | DMF | Cs2CO3 | 10 | 100 | 50 | 92 |
| 6 | DMF | NaOAc | 10 | 100 | 50 | 72 |
| 7 | DMF | NaOH | 10 | 100 | 50 | 63 |
| 8 | DMF | Et3N | 10 | 100 | 50 | 64 |
| 9 | DMF | K2CO3 | None | 100 | 50 | Trace |
| 10 | DMF | K2CO3 | 1 | 100 | 50 | 40 |
| 11 | DMF | K2CO3 | 2 | 100 | 50 | 75 |
| 12 | DMF | K2CO3 | 5 | 100 | 50 | 92 |
| 13 | DMF | K2CO3 | 5 | 80 | 50 | 91 |
| 14 | DMF | K2CO3 | 5 | r.t. | 50 | 68 |
Firstly, the effect of both polar protic and aprotic solvents was studied using K2CO3 as base and in the presence of 10 mol% of cobalt ferrite nanoparticles (entries 1–4). The reaction was also conducted in water as green solvent (entry 3), but the desired yields were not achieved in none of these cases. Therefore, DMF was selected as the optimum solvent (entry 1).
In the next step, for further improving the efficiency of the reaction, various types of organic and inorganic bases were investigated under the same conditions (entries 1, 5–8). In each case, the best results were obtained using both K2CO3 and Cs2CO3 as base. So, considering more availability of K2CO3 beside its lower price, it was employed as the optimum base.
Determination of the amount of catalyst required for the reaction was the next step in this work. For this purpose, the model reaction was carried out in the absence of the catalyst and also in the presence of 1, 2, 5, and 10 mol% of the cobalt ferrite nanocatalyst under the obtained optimized reaction conditions and finally it was demonstrated that use of 5 mol% of catalyst can effectively catalyze the reaction in a short time with a reasonable yield (entries 1, 9–12).
The effect of temperature on the reaction was also examined by performing the model reaction at room temperature, 80 °C and 100 °C (entries 12–14). According to the results listed in Table 1, the best results can be obtained at 80 °C with higher conversion during shorter times. Further increasing the temperature until 100 °C could not increase the rate of reactions or their conversions.
Initially, various types of aryl halides were reacted with different phenol derivatives under the optimized reaction conditions to produce diverse diaryl ethers using magnetic cobalt ferrite nanoparticles.
The generality of this procedure in C–O cross coupling reaction for different aryl halides and alcohols was explored. Fortunately, all types of aryl halides including aryl iodides, bromides and even less reactive aryl chlorides can be employed in this reaction successfully. Using aryl iodides, bromides and chlorides, reactions were proceeded in short times with complete conversions and excellent yields. Also, aryl halides bearing both electron-donating and electron-withdrawing substituents can participate in the reactions. Predictably, the more steric hindrance on aryl halide or alcohol is, the slower the reaction rate between the two coupling partners would be (entries 5, 12, 14, 19, 20 and 25, Table 2). As can be seen in Table 2, the presence of electron withdrawing groups on the phenol ring decreases the reaction rate, while electron donating substituents can accelerate the coupling reaction (entries 6–12 and 19–23, Table 2).
| Entry | Ar–X | R–OH | Product | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction condition: aryl halide (1 mmol), alcohol (1.1 mmol), K2CO3 (1.2 mmol), cobalt ferrite nanoparticles (5 mol%), DMF (2 ml) and 80 °C.b Isolated yield. | |||||
| 1 | Iodobenzene | Phenol |  |
60 | 96 |
| 2 | Bromobenzene | Phenol |  |
80 | 93 |
| 3 | Chlorobenzene | Phenol |  |
100 | 91 |
| 4 | 4-Bromotoluene | Phenol |  |
85 | 89 |
| 5 | 2-Bromo-4-phenyl acetophenone | Phenol |  |
300 | 86 |
| 6 | Iodobenzene | 4-Methoxyphenol |  |
60 | 94 |
| 7 | Bromobenzene | 4-Methoxyphenol |  |
75 | 92 |
| 8 | Chlorobenzene | 4-Methoxyphenol |  |
90 | 81 |
| 9 | 4-Bromobenzonitrile | 4-Methoxyphenol |  |
100 | 91 |
| 10 | 4-Chloroacetophenone | 4-Methoxyphenol |  |
100 | 92 |
| 11 | 4-Bromoanisole | 4-Methoxyphenol |  |
150 | 87 |
| 12 | 4-Acetyl-3-bromonitrobenzene | 4-Methoxyphenol |  |
450 | 83 |
| 13 | Bromobenzene | 1-Naphthol |  |
100 | 90 |
| 14 | 2-Methylbromobenzene | 1-Naphthol |  |
240 | 83 |
| 15 | 4-Bromotoluene | 1-Naphthol |  |
270 | 88 |
| 16 | 4-Chloroacetophenone | 1-Naphthol |  |
250 | 85 |
| 17 | Bromobenzene | 2-Naphthol |  |
150 | 90 |
| 18 | Chlorobenzene | 2-Naphthol |  |
240 | 88 |
| 19 | Bromobenzene | 2-Hydroxybenzaldehyde | 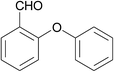 |
270 | 87 |
| 20 | 2-Chlorobenzaldehyde | 2-Hydroxybenzaldehyde |  |
360 | 80 |
| 21 | 4-Bromoanisole | 4-Acetylphenol |  |
150 | 89 |
| 22 | Bromobenzene | 4-Hydroxy-chromen-2-one | 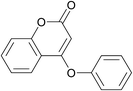 |
120 | 85 |
| 23 | Bromobenzene | 3-Methoxy-phenol |  |
90 | 91 |
| 24 | 4-Bromoanisole | 2-Methylphenol |  |
300 | 87 |
| 25 | 4-Bromoanisole | 4-Allyl-2-methoxyphenol | 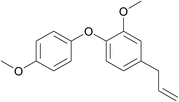 |
360 | 82 |
Considering excellent activity of the cobalt ferrite catalyst, use of nickel ferrite nanoparticles as another spinel bimetallic catalyst in the same reaction and comparison of its activity with cobalt ferrite under similar conditions found to be interesting. So, in the next step, application of NiFe2O4 as catalyst was investigated in the C–O bond formation reaction under the conditions similar to the abovementioned optimized conditions. Results are reported in Table 3. In this case, again all types of aryl halides including aryl iodides, bromides and chlorides bearing both electron-donating and electron-withdrawing groups resulted in the formation of the desired products with yields comparable with the previous catalytic system. However, using this catalyst, reactions required approximately shorter times to be completed indicating better activity of the nickel ferrite nanoparticles compared to cobalt ferrite ones.
| Entry | Ar–X | R–OH | Product | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction condition: aryl halide (1 mmol), alcohol (1.1 mmol), K2CO3 (1.2 mmol), nickel ferrite nanoparticles (5 mol%), DMF (2 ml) and 80 °C.b Isolated yield. | |||||
| 1 | Iodobenzene | Phenol |  |
60 | 96 |
| 2 | Bromobenzene | Phenol |  |
80 | 94 |
| 3 | Chlorobenzene | Phenol | 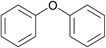 |
90 | 91 |
| 4 | 4-Bromotoluene | Phenol | 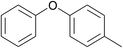 |
75 | 92 |
| 5 | 2-Bromo 4-phenyl acetophenone | Phenol |  |
270 | 85 |
| 6 | Iodobenzene | 4-Methoxyphenol |  |
60 | 93 |
| 7 | Bromobenzene | 4-Methoxyphenol |  |
70 | 92 |
| 8 | Chlorobenzene | 4-Methoxyphenol | 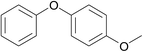 |
90 | 89 |
| 9 | 4-Bromobenzonitrile | 4-Methoxyphenol |  |
95 | 93 |
| 10 | 4-Chloroacetophenone | 4-Methoxyphenol |  |
90 | 92 |
| 11 | 4-Bromoanisole | 4-Methoxyphenol |  |
110 | 90 |
| 12 | 4-Acetyl-3-bromonitrobenzene | 4-Methoxyphenol |  |
420 | 85 |
| 13 | Bromobenzene | 1-Naphthol |  |
100 | 89 |
| 14 | 2-Methylbromobenzene | 1-Naphthol | 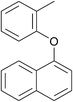 |
210 | 79 |
| 15 | 4-Bromotoluene | 1-Naphthol | 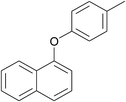 |
240 | 88 |
| 16 | 4-Chloroacetophenone | 1-Naphthol |  |
220 | 86 |
| 17 | Bromobenzene | 2-Naphthol |  |
120 | 90 |
| 18 | Chlorobenzene | 2-Naphthol |  |
180 | 84 |
| 19 | Bromobenzene | 2-Hydroxybenzaldehyde |  |
180 | 83 |
| 20 | 2-Chlorobenzaldehyde | 2-Hydroxybenzaldehyde |  |
270 | 82 |
| 21 | 4-Bromoanisole | 4-Hydroxyacetophenone |  |
100 | 87 |
| 22 | Bromobenzene | 4-Hydroxy-chromen-2-one |  |
100 | 86 |
| 23 | Bromobenzene | 3-Methoxy-phenol |  |
70 | 91 |
| 24 | 4-Bromoanisole | 2-Methylphenol | 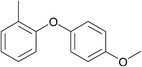 |
200 | 88 |
| 25 | 4-Bromoanisole | 4-Allyl-2-methoxyphenol | 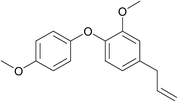 |
240 | 85 |
To check the reusability of the catalyst, the C–O coupling reaction of 4-bromobenzene with phenol was performed under the aforementioned optimized reaction conditions. After the first run, the isolated yield of the reaction was measured exactly; then, ferromagnetic nanoparticles of the catalyst (nickel ferrite or cobalt ferrite) were separated easily by creation of an external magnetic field using a magnet. These isolated nanoparticles after washing with H2O and drying on air were employed in the same reaction for the second run. The isolated yield of the second reaction in each case confirmed that the nanoparticles were not deactivated during the first run of the reaction. Similar results were obtained during the third, fourth, fifth and sixth runs of the reaction and the recycled catalyst in each case could effectively catalyze the reaction without any significant loss of activity (Scheme 2). However, the yield of the reaction using nickel ferrite nanoparticles in the 7th run decreased to 62%. In the case of cobalt ferrite, the first sharp decrease was observed at 9th run resulting in 61% yield of the isolated product.
The SEM images of the recovered catalysts before using in the reaction and after sixth runs of the reactions also were obtained to further confirm that the size, morphology and dispersity of the nanoparticles were not approximately altered during the process (Fig. 1a–d). Fig. 1a and b show the SEM images of the cobalt ferrite nanoparticles before employing as catalyst and after seven time reuse, respectively. Fig. 1c and d also show the SEM images of the nickel ferrite magnetic nanoparticles before using as catalyst and after sixth run of the reaction, respectively. According to the images, it is obvious that the conditions exerted on the reaction media cannot cause to loss of catalytic activity and morphology change.
To investigate the leaching of these catalysts, two similar control experiments were performed in each case. To this end, the reaction between bromobenzene and phenol was carried out simultaneously in two different vessels under the exactly same conditions. In each case, the reaction progress was monitored using gas chromatography after 10 minutes. Then the catalyst's nanoparticles were collected from one of these vessels using a magnet, while the other was remained unchanged. Thereafter, both of the reactions were continued under the same conditions. After another 10 minutes, both the reactions were stopped and their completion was again examined using GC. The results showed that the reactions in the absence of the catalyst were not proceeded any more, while completion of the reaction in the other vessel was increased. This observation confirmed that no leaching was occurred in the reaction medium during the process.
In fact, the leaching phenomenon that often is seen in the heterogeneous catalytic systems does not present here perhaps because of the specific nature of the catalysts. In fact, in these compounds, the whole catalyst is a single spinel structure with a defined lattice where in Fe3+ ions are located in tetrahedral sites and Ni2+/Co2+ ions are in octahedral sites. So, here there is no support and therefore no leaching effect is observed in the formal form. But it is probable that the magnetic properties of the nanoparticles in our case be changed during the reaction resulting in incomplete collection of them in the presence of an external magnetic field. This can cause to loss of catalyst in each experiment.
It is demonstrated that the presence of metal contaminants presented in the iron source can catalyze the coupling reaction between aryl halides and various types of nucleuphiles.34,35 So, two control experiments was done using 5 mol% of FeCl3·6H2O or Fe(NO3)3·9H2O as catalyst between phenol and bromobenzene under the optimized reaction conditions. Having comparable results with those in the presence of nickel ferrite, the reactions were stopped after 80 minutes. The results showed that FeCl3·6H2O/Fe(NO3)3·9H2O is unable to catalyzed the reaction effectively (Scheme 3a and b).
 | ||
| Scheme 3 Control experiments for confirming the essential role of metal ferrite nanoparticles as catalyst. | ||
Two further control experiments were done using NiCl2·6H2O and Co(NO3)2·6H2O as catalyst to confirm the absence of catalytic effect of metal contaminants in the nickel/cobalt sources, respectively. To this end, the reaction between bromobenzene and phenol was repeated under the optimized reaction conditions using 5 mol% of NiCl2·6H2O/Co(NO3)2·6H2O as catalyst. Again, the reactions were stopped after 80 minutes and their conversions were determined exactly. The amount of the desired products in these cases was also very small (Scheme 3c and d).
To investigate the catalytic efficiencies of NiO, CoO and Fe2O3 (metal oxides components of the MFe2O4) in the C–O coupling reaction, each one of them were employed as catalyst in the reaction of bromobenzene and phenol (Scheme 3e–g). After stirring for 80 minutes under the optimized reaction conditions, results confirmed that these metal oxides are also unable to catalyze the reaction efficiently.
Despite the significant development on mixed metal ferrite (MFe2O4) catalyzed cross-coupling reactions, the mechanism of the reaction is still little explored. To explore the exact pathway of the coupling reaction more concern is needed. Obviously, the SNAr mechanism and also reaction through aryne intermediate are not possible in these cases. The evidence for the former claim is that the reaction could not be proceeded without MFe2O4. On the other hand, K2CO3 is not strong enough to remove the ortho-hydrogen and form an aryne intermediate. When the reaction between 4-bromotoluene and phenol is performed, no isomers were obtained; another evidence for the absence of aryne intermediates.
These results along with some recent published works36,37 led us to propose a mechanism for these reactions. It is widely accepted that the first step in this reaction is the oxidative-addition in which MFe2O4 nanoparticles inserts themselves in the Ar–X bonds. The nanoparticles may facilitate the oxidative coupling reaction with an aryl halide due to the easier transfer of electrons and higher surface area in comparison to bulk metal salts. At this stage, we are not sure whether the active catalytic species is M2+ or Fe3+ species or a combination of them. These species undergo oxidative addition step to form intermediate A. Subsequently, reaction of A with the nucleophile gives intermediate B. Reductive-elimination on the intermediate B completes the catalytic cycle producing the desired cross-coupled product C and regenerates the active species of the catalyst (Scheme 4).
 | ||
| Scheme 4 Plausible mechanism of the MFe2O4 (M = Ni or Co) nanoparticle-catalyzed cross-coupling reactions. | ||
According to these, it can be concluded that both proposed catalytic systems not only can catalyze appropriately the reaction, but also can easily be separated and reused for several times without any significant activity lost. In addition, these bimetallic spinel catalysts are cheaper and more effective than previous palladium-based catalytic systems and their raw materials are available.
Experimental
Materials and characterization methods
All materials used are commercially available and were purchased from Merck and used without any additional purification. 1H NMR and 13C NMR spectra were recorded on a Bruker (Avance DRX-500) spectrometer using CDCl3 as solvent at room temperature. Chemical shifts δ were reported in ppm relative to tetramethylsilane as an internal standard. XRF analysis was recorded on Spectro Xepos Spectrometer. The characterization of the samples was done by crystallographic phase identification performed on a Sradi P diffractometer with Cu Kα radiation, a graphite monochromator on the diffracted beam and oscillation counter. FTIR spectra of samples were taken using an ABB Bomem MB-100 FTIR spectrophotometer. Gas chromatography (GC) analyses were performed on an Agilent Technologies 6890 N, equipped with a 19019 J-413 HP-5, 5% phenyl methyl siloxane, capillary column (60.0 m × 250 μm × 1.00 μm). The morphology of the catalyst was observed using a Philips XL30 scanning electron microscope (SEM). Magnetic measurements were carried out at room temperature and by using an AGFM magnetometer.Synthesis of nickel ferrite (NiFe2O4)
The solutions of iron chloride (FeCl3·6H2O) (0.2 M) and nickel chloride (NiCl2·6H2O) (0.1 M) were prepared separately and mixed together. In order for pH to reach 13, a solution of NaOH (3 M) was added slowly to the flask. Finally, oleic acid (3 drops) was added to the solution as surfactant. Then, the suspension was vigorously stirred using a magnetic stirring bar at 60 °C for 2 h. After complete precipitation, the residue was washed with double distilled water (3 × 25 ml) and dried in an oven at 90 °C over night; then it was calcinated at 600 °C for 4 h.Synthesis of cobalt ferrite (CoFe2O4)
The solutions of iron nitrate (Fe(NO3)3·9H2O) (0.2 M) and cobalt nitrate (Co(NO3)2·6H2O) (0.1 M) were prepared separately and mixed together. In order for pH to reach 12, a solution of NaOH (3 M) was added slowly to the flask. Finally, oleic acid (3 drops) was added to the solution as surfactant. Then, the suspension was vigorously stirred using a magnetic stirring bar at 90 °C for 2 h. After complete precipitation, the residue was washed with double distilled water (3 × 25 mL) and dried in an oven at 90 °C over night; then it was calcinated at 600 °C for 5 h.General procedure for the C–O bond formation reaction
In a round-bottom flask equipped with a magnetic stirring bar, aryl halide (1 mmol), aromatic alcohol (1.1 mmol), K2CO3 (1.2 mmol), catalyst (0.05 mmol) and DMF (2 ml) were added and heated at 80 °C. The mixture was vigorously stirred under these reaction conditions and its completion was monitored by TLC (EtOAc–n-hexane, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75).
75).
In each case, after completion of the reaction, the mixture was diluted with ethyl acetate and water. The organic layer was washed with brine (2 × 5 ml), dried over MgSO4, and concentrated under reduced pressure using a rotary evaporator. The residue was purified by recrystallization from ethanol and water or using plate chromatography.
All of the compounds have been characterized by interpreting their 1H and 13C NMR spectra and comparing them with the values found in the literatures (ESI file†).
Conclusion
In summary, we have developed an efficient method for the C–O coupling reaction between various kinds of aromatic alcohols and aryl halides. Two different magnetic spinel catalysts were synthesized and their activities in the C–O bond formation reaction were compared. These nanoparticles are easily made, air-stable, of low cost, and magnetic. They can be easily recycled using an external magnetic field. Reactions were completed in reasonably short reaction times with very good to excellent yields. Comparison of these two catalytic system shows that their activity is very close to each other; however, nickel ferrite nanoparticles could catalyzed the reactions in approximately shorter reaction times compared to cobalt ferrite ones.Acknowledgements
We gratefully acknowledge the funding support received for this project from the Sharif University of Technology (SUT), Islamic Republic of Iran.References
- (a) F. Monnier and M. Taillefer, Angew. Chem., 2009, 48, 6954–6971 CrossRef CAS PubMed; (b) A. Biffis, M. Gardan and B. Corain, J. Mol. Catal. A: Chem., 2006, 250, 1–5 CrossRef CAS PubMed; (c) S. G. Babu and R. Karvembu, Tetrahedron Lett., 2013, 54, 1677–1680 CrossRef CAS PubMed; (d) J. S. Sawyer, Tetrahedron, 2000, 56, 5045–5065 CrossRef; (e) E. Sperotto, G. P. M. v. Klink, J. G. de Vries and G. v. Koten, Tetrahedron, 2010, 66, 9009–9020 CrossRef CAS PubMed.
- G. Song, F. Wang and X. Li, Chem. Soc. Rev., 2012, 41, 3651–3678 RSC.
- G. Pandey, S. Pal and R. Laha, Angew. Chem., 2013, 52, 5146–5149 CrossRef CAS PubMed.
- X. Lv and W. Bao, J. Org. Chem., 2007, 72, 3863–3867 CrossRef CAS PubMed.
- S. Jammi, S. Sakthivel, L. Rout, T. Mukherjee, S. Mandal, R. Mitra, P. Saha and T. Punniyamurthy, J. Org. Chem., 2009, 74, 1971–1976 CrossRef CAS PubMed.
- S. Gowrisankar, A. G. Sergeev, P. Anbarasan, A. Spannenberg, H. Neumann and M. Beller, J. Am. Chem. Soc., 2010, 132, 11592–11598 CrossRef CAS PubMed.
- D. Kundu, P. Maity and B. C. Ranu, Org. Lett., 2014, 16, 1040–1043 CrossRef CAS PubMed.
- J. P. Finet, A. Y. Feclorov, S. Combes and G. Boyer, Curr. Org. Chem., 2002, 6, 597–626 CrossRef CAS.
- S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400–5449 CrossRef CAS PubMed.
- K. Kunz, U. Scholz and D. Ganzer, Synlett, 2003, 2428–2439 CrossRef CAS.
- I. P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337–2364 CrossRef CAS PubMed.
- R. Frlan and D. Kikelji, Synthesis, 2006, 2271–2458 CAS.
- (a) D. A. Evans, M. R. Wood, B. W. Trotter, T. I. Richardson, J. C. Barrow and J. L. Kartz, Angew. Chem., Int. Ed., 1998, 37, 2700–2704 CrossRef CAS; (b) D. L. Boger, S. Miyazaki, S. H. Kim, J. H. Wu, S. L. Castle, O. Loiseleur and Q. Jin, J. Am. Chem. Soc., 1999, 121, 10004–10011 CrossRef CAS; (c) D. J. Procter, J. Chem. Soc., Perkin Trans. 1, 2001, 335–354 RSC.
- D. A. Evans, C. J. Dinsmore, P. S. Watson, M. R. Wood, T. I. Richardson, B. W. Trotter and J. L. Katz, Angew. Chem., Int. Ed., 1998, 37, 2704–2708 CrossRef CAS.
- F. B. Belval, A. Rouch, C. V. Bacqué and M. Baltas, Med. Chem. Commun., 2012, 3, 1356–1372 RSC.
- (a) R. Frlan and D. Kikelj, Synthesis, 2006, 2271–2285 CAS; (b) J. Niu, H. Zhou, Z. Li, J. Xu and S. Hu, J. Org. Chem., 2008, 73, 7814–7817 CrossRef CAS PubMed.
- (a) J. Lindley, Tetrahedron, 1984, 40, 1433–1456 CrossRef CAS; (b) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054–3131 CrossRef CAS PubMed.
- M. Kidwai, N. K. Mishra, V. Bansal, A. Kumar and S. Mozumdar, Tetrahedron Lett., 2007, 48, 8883–8887 CrossRef CAS PubMed.
- (a) J. P. Wolfe, S. Wagaw, J.-F. O. Marcoux and S. L. Buchwald, Acc. Chem. Res., 1998, 31, 805–818 CrossRef CAS; (b) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417–1492 CrossRef CAS PubMed; (c) M. Beller and C. Bolm, Transition Metals for Organic Synthesis: Building Blocks and Fine Chemicals, New York, 2008 Search PubMed; (d) R. Bates, Organic Synthesis Using Transition Metals, New York, 2012 Search PubMed.
- (a) I. Beletskaya and V. Tyurin, Molecules, 2010, 15, 4792–4814 CrossRef CAS PubMed; (b) B. P. S. Chauhan, R. Thekkathu, K. L. Prasanth, M. Mandal and K. Lewis, Appl. Organomet. Chem., 2010, 24, 222–228 CrossRef CAS PubMed; (c) B. P. S. Chauhan, R. Sardar, U. Latif, M. Chauhan and W. Lamoreaux, Acta Chim. Slov., 2005, 52, 361–370 CAS.
- N. Toshima and T. Yonezawa, New J. Chem., 1998, 22, 1179–1201 RSC.
- Y. Kinemuchi, K. Ishizaka, H. Suematsu, W. Jiang and K. Yatsui, Thin Solid Films, 2002, 407, 109 CrossRef CAS.
- K. Sue, M. Aoki, T. Sato, D. N. Hamane, S. Kawasaki, Y. Hakuta, Y. Takebayashi, S. Yoda, T. Furuya, T. Sato and T. Hiaki, Ind. Eng. Chem. Res., 2011, 50, 9625–9631 CrossRef CAS.
- C. M. Lukehart and R. A. Scott, Nanomaterials: Inorganic and Bioinorganic Perspectives, John Wiley & Sons, 2008 Search PubMed.
- S. Ammar, A. Helfen, N. Jouini, F. Fiévet, I. Rosenman, F. Villain, P. Molinié and M. Danot, J. Mater. Chem., 2001, 11, 186–192 RSC.
- F. M. Moghaddam, G. Tavakoli and H. R. Rezvani, Appl. Organomet. Chem., 2014, 28, 750–755 CrossRef PubMed.
- F. M. Moghaddam, G. Tavakoli, A. Moafi, V. Saberi and H. R. Rezvani, ChemCatChem, 2014, 6, 3474–3481 CAS.
- F. M. Moghaddam, G. Tavakoli and H. R. Rezvani, Catal. Commun., 2015, 60, 82–87 CrossRef CAS PubMed.
- (a) M. Akkoça, N. Gürbüza, E. Çetinkayab and I. Özdemir, Synlett, 2008, 1781–1784 Search PubMed; (b) Y. Liu and S. Zhang, Synlett, 2011, 268–272 CAS; (c) Y.-J. Chen and H.-H. Chen, Org. Lett., 2006, 8, 5609–5612 CrossRef CAS PubMed; (d) D. Ma and Q. Cai, Org. Lett., 2003, 5, 3799–3802 CrossRef CAS PubMed.
- S. Yang, C. Wu, H. Zhou, Y. Yang, Y. Zhao, C. Wang, W. Yang and J. Xu, Adv. Synth. Catal., 2013, 355, 53–58 CrossRef CAS PubMed.
- C. Qian, L. Qin, Q. Zong, L. Wu and D. Fang, Bull. Korean Chem. Soc., 2013, 34, 3915–3918 CrossRef CAS.
- M. A. Khalilzadeh, H. Keipour, A. Hosseini and D. Zareyee, New J. Chem., 2014, 38, 42–45 RSC.
- Y. Zhang, Z. Yang, D. Yin, Y. Liu, C. L. Fei, R. Xiong, J. Shi and G. L. Yan, J. Magn. Magn. Mater., 2010, 322, 3470–3475 CrossRef CAS PubMed.
- S. L. Buchwald and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5586 CrossRef CAS PubMed.
- Z. Gonda, G. L. Tolnai and Z. Novák, Chem.–Eur. J., 2010, 16, 11822 CrossRef CAS PubMed.
- S. Yang, C. Wu, H. Zhou, Y. Yang, Y. Zhao, C. Wang, W. Yang and J. Xu, Adv. Synth. Catal., 2013, 355, 53–58 CrossRef CAS PubMed.
- S. Paul, K. Pradhan, S. Ghosh, S. K. De and A. R. Das, Adv. Synth. Catal., 2014, 356, 1301–1316 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra08146g |
| This journal is © The Royal Society of Chemistry 2015 |