White organic light emitting diodes based on a yellow thermally activated delayed fluorescent emitter and blue fluorescent emitter†
Abstract
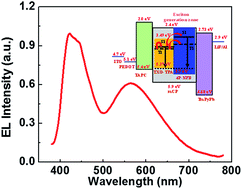
Multi-emissive layered white organic light-emitting diodes (WOLEDs) based on a blue fluorescent emitter and a yellow thermally activated delayed fluorescent emitter were constructed with the co-host of mCP. The WOLEDs afforded a color coordination of (0.34, 0.34), low efficiency roll-off, and a maximum external quantum efficiency of 4.7%.


 Please wait while we load your content...
Please wait while we load your content...