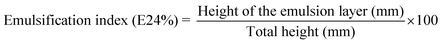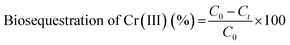Biosequestration of chromium(III) in an aqueous solution using cationic and anionic biosurfactants produced from two different Bacillus sp. – a comparative study†
P. Saranya,
P. Bhavani,
S. Swarnalatha and
G. Sekaran*
Environmental Technology Division, CSIR-Central Leather Research Institute (CLRI), Adyar, Chennai, India. E-mail: ganesansekaran@gmail.com; Fax: +91-44-24410232; Tel: +91-44-24452941
First published on 9th September 2015
Abstract
Tannery wastewater discharged from chrome tanning section contains Cr(III) in the range of 2100–2300 ppm, and a viable technique for its removal remains a great concern for the leather industry. The ability of a biosurfactant to chelate toxic heavy metal ions and form an insoluble precipitate may be exploited in the treatment of Cr(III) containing wastewater. In the present study, a biosurfactant producing microorganisms, Bacillus subtilis and Bacillus cereus, were isolated from tannery wastewater-contaminated soil using palm oil and coconut oil as the substrates. The biosurfactants produced from palm oil (palm oil BS) and coconut oil (coconut oil BS) were characterized as anionic and cationic biosurfactants, respectively, using the blue agar plate method, agar double diffusion technique and zeta potential measurement. The biosurfactants were characterized for their amino acid composition and elemental (CHNS) composition. The thermal behavior of the biosurfactant was characterized by TGA and DSC. The surface tension of the anionic and cationic biosurfactants were 28.16 ± 0.2 mN m−1 and 23.02 ± 0.2 mN m−1, respectively. The SDS-PAGE and FT-IR analyses confirmed that both the biosurfactants were lipoproteins in nature. The binding ability of the lipoprotein anionic and lipoprotein cationic BS with chromium (Cr(III)) ions in an aqueous solution was then determined. The interaction of Cr(III) with BS was confirmed using FT-IR, SEM-EDX analysis and atomic absorption spectrophotometry (AAS).
1. Introduction
The leather manufacturing industry converts putrescible raw skins and hides into non putrescible leather through various chemical and mechanical processes.1 Basic chromium sulphate is the most common tanning agent used in the leather industry because it enables the faster and cheaper production of highly microbial resistant and durable leather. More than 80% of finished leather goods are tanned using basic chromium sulphate.2 The amount of trivalent chromium discharged in the effluent, when processing one ton of wet salted skins/hides, is in the range of 3–7 kg or 5–10 kg Cr2O3.3 According to the available reports,4 the recommended level for Cr(III) is in the range of 0.5–2.0 ppm regardless of fresh, marine, irrigation and drinking water. In many countries, the treated tannery wastewater is discharged onto the open land or into rivers.5Cr(III) tends to be strongly bound by soil humic acid polymers, and this affinity restricts the availability of Cr(III) to be oxidized and reduces the decomposition of organic matter.6 Cr(III) in soils could be leached into surface water or groundwater and be absorbed by plants.5 Chromium(III) enters the human food chain through the consumption of the vegetative plants. The symptoms of Cr(III) phytotoxicity include inhibition of seed germination or of early seedling development, reduction of root growth, leaf chlorosis and depressed biomass.7
Tannery wastewater from the chrome tanning section contains Cr(III) in the range of 2100–2300 ppm.8 The improper treatment of Cr(III) containing effluent, non scientific method of storage of chromium tanned solid waste and the leaching of Cr(III) onto the soil can release Cr(III) to the environment, causing groundwater contamination and adverse biological and ecological effects.9 Precipitated Cr(III) hydroxides remain stable in the sediments under aerobic conditions, whereas under acidic and anoxic conditions, Cr(III) hydroxides are soluble and remain as ionic Cr(III) species,10 which can leach into the groundwater sources.
Cleaner technologies used to reduce Cr(III) in wastewater, such as high exhaustion process and direct or indirect chromium recycling, cannot eliminate Cr(III) completely from tannery wastewater.11 Among the techniques known for the removal of chromium from tannery wastewater, the biosequestration of Cr(III) is one of the proven technologies.
Biosurfactants are a structurally diverse group of surface-active substances produced by microorganisms. They consist of a polar hydrophilic moiety and a non-polar hydrophobic group. The hydrophilic group consists of mono, oligo or polysaccharides, peptides or proteins, and the hydrophobic moiety contains saturated, unsaturated and hydroxylated fatty acids or fatty alcohols.12,13 The six major types of biosurfactants available are hydroxylated and cross linked fatty acids (mycolic acids), glycolipids, lipopolysaccharides, lipoproteins and phospholipids.14 Biosurfactants are used in the remediation of pollutants due to their surface active properties.15
The chemical configuration of biosurfactants allows them to bind with the metal ions. The characteristic feature of biosurfactants to chelate toxic heavy metals and form an insoluble precipitate may be exploited in the treatment of heavy metal containing wastewater.16 Biosurfactants of anionic nature can capture metal ions through electrostatic interactions or complexation. Cationic biosurfactants can replace the same charged metal ions by competition.17 To the best of our knowledge, there are no reports on the removal of Cr(III) using cationic biosurfactants.
The focal theme of the present investigation was to compare the Cr(III) binding ability of anionic and cationic biosurfactants (BS) produced from two different substrates such as palm oil and coconut oil.
2. Materials and methods
2.1. Isolation of microorganisms
Biosurfactants producing microorganisms were isolated from tannery wastewater contaminated soil. The soil was acclimatized with palm oil or coconut oil and serially diluted to isolate the microorganisms using nutrient agar by the pour plate method, followed by incubation for 24–48 h at 35 °C.2.2. Screening and identification of biosurfactant producing microorganisms
The surface active properties of the biosurfactant produced by the isolated bacterial strains were screened by their oil drop collapse activity17 using four different oils, namely, palm oil, diesel oil, coconut oil, and olive oil. The organisms screened from palm oil and coconut oil acclimatized soil were identified by 16S ribosomal DNA (16S rDNA) sequencing and phylogenetic analyses.2.3. Optimization studies for the production of biosurfactants
The various parameters that significantly affect the production of biosurfactants using palm oil and coconut oil as substrates, such as time (24–120 h), pH (1–10), temperature (20–50 °C) and concentration of substrate (10–60 g L−1), were optimized using one parameter at a time while the other parameters were kept constant. The most significant range of parameters was further optimized using Response Surface Methodology (RSM) for the production of the biosurfactant.2.4. Production, extraction and purification of biosurfactants
The production of BS was carried out under optimized conditions and the bacterial cells were removed by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min at 4 °C. The cell free supernatant was adjusted using 2 N HCl to obtain the final pH to 2.0 and kept for overnight at 4 °C. The pellet was collected by centrifugation at 9000 rpm for 20 min at 4 °C and the biosurfactant was extracted with acetone. The extracted biosurfactant was purified further using silica gel column chromatography to obtain the purified biosurfactant.
000 rpm for 20 min at 4 °C. The cell free supernatant was adjusted using 2 N HCl to obtain the final pH to 2.0 and kept for overnight at 4 °C. The pellet was collected by centrifugation at 9000 rpm for 20 min at 4 °C and the biosurfactant was extracted with acetone. The extracted biosurfactant was purified further using silica gel column chromatography to obtain the purified biosurfactant.
2.5. Characterization of biosurfactant
Blue agar plate method. The ionic nature of biosurfactant was characterized by a slight modification of the blue agar plate method.18 Mineral salt agar medium supplemented with glucose as the carbon source (2%) and methylene blue (MB: 0.2 mg mL−1) were used for the preparation of blue agar plate. The anionic and cationic nature of BS was identified by adding cetyl trimethyl ammonium bromide (CTAB: 0.5 mg mL−1) and sodium dodecyl sulphate (SDS: 0.5 mg mL−1) to the respective media. Biosurfactant (50 μL) containing 10 μg was loaded into each well made in blue agar plate and the plates were incubated at 37 °C for 48–72 h. A dark blue halo zone was considered as the positive result for anionic and cationic biosurfactants in their corresponding plates.
Agar double diffusion technique. The ionic character of BS was determined using the agar double diffusion technique.19 In an agar plate (1% agar of low hardness), two regularly-spaced rows of wells were made. The purified BS of volume 100 μL containing 20 μg was filled in the wells in the lower row, whereas the upper row of the well was filled with a pure compound (SDS or CTAB) of a known ionic charge. The anionic and cationic nature of BS was identified by adding CTAB (0.5 mg mL−1) and SDS (0.5 mg mL−1) in the wells of the upper row. The appearance of precipitation lines between the corresponding wells is considered to be due to the ionic nature of the biosurfactant.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min and then the surface tension of the supernatant was measured.21 Surface tension measurements were recorded by the Wilhelmy Plate method using a Surface Tensiometer (NIMA Technologies Ltd., England).
000 rpm for 20 min and then the surface tension of the supernatant was measured.21 Surface tension measurements were recorded by the Wilhelmy Plate method using a Surface Tensiometer (NIMA Technologies Ltd., England).2.6. Amino acid composition of biosurfactant by HPLC
The amino acid composition of the biosurfactant was determined by HPLC. The biosurfactant was hydrolyzed with 6 N HCl at 100 °C for 20 h and neutralized with 1 M NaOH. The amino acid composition was analyzed using an Agilent 1100 HPLC amino acid analyzer (Agilent Technologies, Middleburg, Netherlands) and a data analysis was performed using the HP chemstation.252.7. Molecular weight determination of biosurfactant
The molecular mass of the biosurfactant was determined by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), in accordance with the method of Laemmli,26 on a 5% stacking gel and 12% resolving gel. The protein marker ranging from 14.3 to 94.7 KDa was used as a standard marker for the determination of molecular weight of the biosurfactant.2.8. Instrumental characterization of biosurfactant
For DSC analysis, the BS samples (8–10 mg) were loaded in an aluminum DSC pan and gravimetric analysis was performed under a nitrogen atmosphere from 0 °C to 200 °C using a temperature gradient of 10 °C min−1. The scans were routinely recorded in duplicate using a DSC Q200 (V23.10 Build 79).
2.9. Interaction of chromium(III) ions with biosurfactants
The biosequestration of chromium(III) ions from an aqueous solution was confirmed by atomic absorption spectroscopy analysis (AAS). The effect of time (2–12 h), pH (4–6), different concentrations of anionic and cationic biosurfactants (0.5, 1.0, 1.5, 2.0 and 2.5 g) and concentration of Cr(III) (100–500 mg L−1) were studied. The time study was performed using 0.5 g anionic and cationic BS for the removal of Cr(III) from the aqueous solution containing 100 mg L−1 Cr(III). Under the optimized conditions, the concentration of Cr(III) before and after sequestration by biosurfactants were measured using an atomic absorption spectrophotometer (AAS).C0 = initial concentration of Cr(III) in mg L−1; Ct = final concentration of Cr(III) in mg L−1.
2.10. UV-visible spectroscopy
The UV-visible scans of cationic BS, anionic BS and BS after the interaction with Cr(III) were recorded in the range of λ200–800 nm using a UV-visible spectrophotometer (Cary varion; Agilent Technologies, Middleburg, Netherlands).2.11. Fluorescence spectroscopy
The fluorescence spectra of cationic BS, anionic BS and BS after the interaction with Cr(III) were recorded using a fluorescence spectrophotometer in the wavelength range of λ200–800 nm (Cary Eclipse; Agilent Technologies, Middleburg, Netherlands).2.12. FT-IR spectral analysis
The functional groups of anionic and cationic lipoprotein BS, before and after treating with Cr(III), were characterized using a FT-IR spectrophotometer (Perkin Elmer). The samples were dried and made in the form of pellet with dimensions thickness of 1 mm and a diameter of 13 mm using spectroscopic grade KBr. The spectra were obtained in the spectral range of 400–4000 cm−1.2.13. SEM analysis
The binding of chromium ions with BS was further confirmed by SEM analysis. The biosurfactant and chromium bound biosurfactant [BS-Cr(III)] samples were coated with a gold foil 120–130 μm in thickness, under an argon atmosphere. SEM images were recorded on a scanning device attached to a JEOL JM-5600 electron microscope at 20 kV accelerating voltage with a 5–6 nm electron beam.2.14. EDX analysis
The biosurfactant and chromium bound biosurfactant [BS-Cr(III)] samples were coated with a gold foil, 120–130 μm in thickness under an argon atmosphere. The EDX spectra were obtained on a scanning device attached to a JEOL JM-5600 electron microscope at a 15 kV accelerating voltage with a 5–6 nm electron beam.3. Results and discussion
3.1. Isolation, screening and identification of biosurfactant producing microorganism
The enrichment of bacteria isolated from the tannery wastewater contaminated soil was carried out in 100 mL M9 Minimal media (HiMedia) with palm oil or coconut oil (1 g) as the sole carbon source. The palm/coconut oil concentration in the media was increased from 1 to 5 g with an incremental increase of 1 g. The media was acclimatized for 4–5 weeks and the microbes were then serially diluted (10−1 to 10−10). The biosurfactant producing microorganisms with a non-identical morphology were isolated from palm oil or coconut oil acclimatized soil. About 6 biosurfactant producing microorganisms from palm oil as the substrate and 4 microorganisms from coconut oil as the substrate were isolated. Based on their zone diameter in the oil drop collapse assay (Table 1), microorganisms with high biosurfactant activity (P3 & C2) were used for further studies. The screened microorganisms were identified as Bacillus sp., using 16s rDNA gene sequencing. Phylogenetic analysis shows the evolutionary relationships among various other Bacillus species based upon the similarities and differences in their physical or genetic characteristics. The phylogenetic trees for the identified organisms are presented in Fig. 1 and confirmed that they belong to Bacillus subtilis and Bacillus cereus.| Palm oil | Coconut oil | ||
|---|---|---|---|
| Isolated microorganisms | Zone diameter (cm) | Isolated microorganisms | Zone diameter (cm) |
| P1 | 0.9 ± 0.5 | C1 | 1.5 ± 0.4 |
| P2 | 1.2 ± 0.4 | C2 | 3.4 ± 0.15 |
| P3 | 2.8 ± 0.3 | C3 | 0.6 ± 0.08 |
| P4 | 0.6 ± 0.2 | C4 | 1.8 ± 0.21 |
| P5 | 1.6 ± 0.4 | ||
| P6 | 1.3 ± 0.3 | ||
 | ||
| Fig. 1 Phylogenetic tree for the organisms identified from (a) palm oil substrate, (b) coconut oil substrate. | ||
3.2. Statistical optimization for the production of biosurfactant
The production of biosurfactants from Bacillus sp. was studied in the batch mode. The process parameters, such as time (24–120 h), pH (1–10), temperature (20–50 °C) and concentration of substrate (10–60 g L−1), were optimized using one parameter at a time. The most significant range of parameters was further optimized using RSM (Refer appendix).RSM clearly states that the biosurfactant production from B. subtilis and B. cereus was significantly (p < 0.05) influenced by pH, temperature and concentration of oil. Nonlinear relationships were significantly (p < 0.05) fitted to the experimental data for describing the changes in biosurfactant yield, when all the experimental variables were simultaneously altered. Among the response variable effects, the interaction effect of pH and palm oil concentration had the most significant (p < 0.05) positive effect on the biosurfactant production from B. subtilis, whereas the interaction effect of temperature and coconut oil concentration had the most significant (p < 0.05) positive effect on the biosurfactant production from B. cereus.
Bacillus subtilis produced BS using 0.32 g per gram of palm oil (12.8 g L−1) as the substrate under the optimized conditions: time, 96 h; pH, 5; temperature, 30 °C; and concentration of palm oil, 40 g L−1. The production of rhamnolipid biosurfactant using palm oil was reported by Thaniyavarn et al. (2.91 g L−1)16 and by Nawawi (85 g L−1) using palm oil sludge.27 Similarly, Bacillus cereus yielded 0.45 g of BS per gram of coconut oil (22.5g L−1) as the substrate under the optimized conditions: time, 96 h; pH, 7; temperature, 40 °C; and concentration of coconut oil, 50 g L−1. The rhamnolipid biosurfactant produced using coconut oil was reported by Thaniyavarn et al. (2.93 g L−1)16 and Patil et al. (2.8 g L−1).28 Kannahi and Sherley29 also reported rhamnolipid biosurfactant of 44.2 g L−1 using mixed oil containing 2% coconut oil.
where k is the rate constant, A is the Arrhenius factor and Ea is the activation energy. The activation energy for the BS production from B. subtilis and B. cereus was calculated from a plot of ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k versus 1/T. The activation energy (Ea) for the production of BS from B. subtilis and B. cereus was found to be 80.59 kJ mol−1 and 87.95 kJ mol−1, respectively. The Arrhenius factor was observed to be 6.88 × 1011 and 1.46 × 1013 h−1 for the production of BS from B. subtilis and B. cereus, respectively.
k versus 1/T. The activation energy (Ea) for the production of BS from B. subtilis and B. cereus was found to be 80.59 kJ mol−1 and 87.95 kJ mol−1, respectively. The Arrhenius factor was observed to be 6.88 × 1011 and 1.46 × 1013 h−1 for the production of BS from B. subtilis and B. cereus, respectively.3.3. Characterization of biosurfactant
Blue agar plate method. The anionic and cationic BS were characterized by the formation of insoluble ion pair precipitates in an agar plate containing methylene blue exhibited in dark blue color against the light blue background. The biosurfactants produced from palm oil and coconut oil as the substrates exhibited a dark blue halo zone in CTAB (positively charged surfactant) and in SDS (negatively charged surfactant) containing plates, respectively (Fig. 2(I)). These results confirm that the biosurfactant from the palm oil substrate was anionic in nature and the biosurfactant from the coconut oil substrate was cationic in nature.30
 | ||
| Fig. 2 (I) Blue agar plate method (a) CTAB plate (anionic BS) (b) SDS plate (cationic BS); (II) double diffusion on Agar (a) CTAB (b) SDS. | ||
Double diffusion agar method. Agar double diffusion tests are based on the passive diffusion of two compounds bearing similar charges or opposite charges in a weakly concentrated gel. Fig. 2(II)a suggests that the precipitation lines were formed between the BS from palm oil and the cationic compound (CTAB), whereas no precipitation was found with BS from coconut oil. Similarly, the precipitation lines were observed between BS from the coconut oil and the anionic compound (SDS), while no precipitation line was observed between SDS and BS from palm oil (Fig. 2(II)b). Therefore, the results confirm that the biosurfactants produced from B. subtilis and B. cereus were anionic and cationic in nature, respectively.
3.3.2. Zeta potential measurement of biosurfactants
Fig. 3 shows that the zeta potential of the coconut oil BS was +54.7 mV and palm oil BS was −33.01 mV. The zeta potential suggests the polarised nature of the surfactants and the degree of polarization. Furthermore, because of the high positive zeta potential, the cationic BS can be expected to enhance the exchange of Cr(III) ions from the aqueous solution compared to the anionic BS.| Hydrocarbons | Emulsification stability index, E24 (%) | |
|---|---|---|
| Palm oil BS | Coconut oil BS | |
| Palm oil | 35 ± 1.2 | 40 ± 1.5 |
| Coconut oil | 30 ± 1.0 | 40 ± 1.0 |
| Olive oil | 22 ± 1.8 | 35 ± 1.5 |
| Diesel oil | 35 ± 1.5 | 38 ± 1.8 |
3.4. Determination of amino acid composition of BS by HPLC
The amino acid composition showed that the anionic lipoprotein BS contained about 61.5% of polar amino acids and 38.5% of non-polar amino acids. The cationic lipoprotein BS contained 53% polar amino acids and 47% non-polar amino acids (Table 3).| Amino acids | μmol g−1 | |
|---|---|---|
| Anionic BS | Cationic BS | |
| Aspartic acid | — | 10.9 |
| Glutamic acid | 23 | 45 |
| Serine | 7.4 | 5.1 |
| Histidine | — | 0.9 |
| Glycine | 0.44 | 8.5 |
| Threonine | 4.23 | 2.6 |
| Arginine | 34.5 | 13.8 |
| Alanine | 3.75 | 6.5 |
| Tyrosine | 1.58 | 15.5 |
| Methionine | 0.14 | 1.6 |
| Valine | 1.64 | 3.1 |
| Phenylalanine | — | 2.9 |
| Isoleucine | — | 2.6 |
| Leucine | 11.3 | 4.5 |
| Lysine | — | 2.2 |
| Cysteine | 2.85 | — |
| Glutamine | 1.22 | — |
| Asparagine | 0.85 | — |
3.5. Molecular weight determination of biosurfactants
The molecular weight of the purified lipoprotein BS was confirmed by SDS-PAGE. The molecular weights of anionic and cationic BS were 18 kDa and 90 KDa, respectively (Fig. 4). The high molecular weight of the biosurfactants confirms that both the biosurfactants were giant molecular lipoproteins. BS from the coconut oil substrate was a high molecular weight lipoprotein compared to the other biosurfactants from other sources reported in the literature.30,35 The reported research on Bacillus subtilis strain indicated that the production of low molecular weight biosurfactant32 and BS from Bacillus cereus strain was reported to produce plipastatins, a family of lipopeptides.36 | ||
| Fig. 4 SDS PAGE for the lipoprotein biosurfactant Lane 1: marker, Lane 2: anionic BS-18 kDa, Lane 3: cationic BS-90KDa. | ||
3.6. Instrumental characterization of BS
TGA of cationic BS showed an initial weight loss of 2.42% at 81.97 °C due to the removal of moisture. The maximum weight loss in BS of 52.25% was due to the decomposition of its constituents over the temperature range from 255.59 °C to 408.74 °C. At the end of the scan, 37.7% of BS remained as a fixed residue at 800 °C. DTG showed major weight loss of 0.4376%/°C at 353.59 °C (Fig. 5b). This suggests that cationic BS was thermally stable compared to anionic BS.
Differential Scanning Calorimetry was used to characterize the phase transition in lipoprotein over the temperature range of 30–300 °C. The transition, which is an exothermic process, was from an amorphous solid to a crystalline solid. The DSC thermogram of anionic BS showed a sharp crystallization temperature at 197 °C (Fig. 6a). The DSC thermogram of cationic BS showed transition temperatures at 107 °C and 197.70 °C (Fig. 6b).
3.7. Interaction of trivalent chromium ions with biosurfactant
3.8. Instrumental evidence of the removal of Cr(III) ions using BS
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the peptide group was observed at 1651.96 cm−1. The peak at 2924.23 cm−1 resulting from the –C–H stretching of the methylene group reflects the presence of an aliphatic chain. The peak at 1466.10 cm−1 was due to the methylene bending vibration. The absorption peak at 1750.11 cm−1 may be due to the presence of carbonyl stretching in the ester groups. The shouldering at 725 cm−1 may correspond to the C–N stretching vibration. This may be due to the bonding of the protein moiety with the lipid component in lipoprotein BS.24 The peaks at 702 and 2511 cm−1 may be due to the C–S and S–H stretching, respectively, of cysteine present in lipoprotein.
O stretching vibration of the peptide group was observed at 1651.96 cm−1. The peak at 2924.23 cm−1 resulting from the –C–H stretching of the methylene group reflects the presence of an aliphatic chain. The peak at 1466.10 cm−1 was due to the methylene bending vibration. The absorption peak at 1750.11 cm−1 may be due to the presence of carbonyl stretching in the ester groups. The shouldering at 725 cm−1 may correspond to the C–N stretching vibration. This may be due to the bonding of the protein moiety with the lipid component in lipoprotein BS.24 The peaks at 702 and 2511 cm−1 may be due to the C–S and S–H stretching, respectively, of cysteine present in lipoprotein.
 | ||
| Fig. 11 FTIR spectra of (a) anionic BS, (b) anionic BS with Cr(III) ions, (c) cationic BS and (d) cationic BS with Cr(III) ions. | ||
The FT-IR spectrum of cationic BS (Fig. 11c) showed N–H stretching of the peptide bond at 3297.26 cm−1 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the peptide group at 1658.41 cm−1. The band at 2952.52 cm−1, resulting from the C–H stretching of methylene group, reflects the presence of an aliphatic chain. The peak at 1465.90 cm−1 was due to methylene bending. The absorption region at 1743.28 cm−1 was due to the carbonyl stretching of ester group. The shouldering at 1107, 1156, 1121, and 1248.28 cm−1 may correspond to the C–N stretching vibrations. The presence of a peptide bond with esters confirms that the biosurfactants were lipoproteins. The characteristic stretching frequency of amides in the regions from 3350 cm−1 to 1500–1650 cm−1 are not normally observed in the FT-IR spectra of rhamnolipid biosurfactants, which differentiates the unique nature of lipoprotein biosurfactant from rhamnolipid biosurfactant.31
O stretching of the peptide group at 1658.41 cm−1. The band at 2952.52 cm−1, resulting from the C–H stretching of methylene group, reflects the presence of an aliphatic chain. The peak at 1465.90 cm−1 was due to methylene bending. The absorption region at 1743.28 cm−1 was due to the carbonyl stretching of ester group. The shouldering at 1107, 1156, 1121, and 1248.28 cm−1 may correspond to the C–N stretching vibrations. The presence of a peptide bond with esters confirms that the biosurfactants were lipoproteins. The characteristic stretching frequency of amides in the regions from 3350 cm−1 to 1500–1650 cm−1 are not normally observed in the FT-IR spectra of rhamnolipid biosurfactants, which differentiates the unique nature of lipoprotein biosurfactant from rhamnolipid biosurfactant.31
Fig. 11b shows the FT-IR spectrum of BS-Cr(III) due to the binding of Cr(III) with the NH group and thus the N–H stretching band at 3451.03 cm−1 was shifted to 3395 cm−1. The shift in frequency to a lower value confirms that a stabilized bond formed between Cr(III) and the NH stretching of anionic BS. The characteristic peak of Cr(III) was observed at 620 cm−1. The C–N stretching vibration was shifted from 725 cm−1 to 713 cm−1 after the ionic bonding of Cr(III) with anionic BS.
The FT-IR spectrum in Fig. 11d showed a broadening of the OH stretching and shifting of amide bond from 3297.26 cm−1 to 3139 cm−1 due to the strong binding of Cr(III). The characteristic peak of Cr(III) was observed at 620 cm−1. The shift in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the peptide group from 1658.41 cm−1 to 1651.41 cm−1 was observed due to the strong binding of cationic biosurfactant with chromium(III) ions. In addition, the band at 1746 cm−1 due to C–O stretching was shortened. This becomes an evidence for the binding of Cr(III) ions with the peptide group of the biosurfactant.
O stretching vibration of the peptide group from 1658.41 cm−1 to 1651.41 cm−1 was observed due to the strong binding of cationic biosurfactant with chromium(III) ions. In addition, the band at 1746 cm−1 due to C–O stretching was shortened. This becomes an evidence for the binding of Cr(III) ions with the peptide group of the biosurfactant.
3.9. Mechanistic view for removal of Cr(III) ions in an aqueous solution by anionic and cationic BS
The biosurfactant acquired cationic charge (+54.7 mV as evidenced from Fig. 3 relating zeta potential profile) due to the presence of a tertiary amine in histidine, resonance structure of phenylalanine and tyrosine in lipoprotein. The amino acids, such as histidine (pI 7.59), phenylalanine (pI 5.91) and tyrosine (pI 5.66), in cationic BS have isoelectric points much higher than the pH of BS (pH 5). At the optimized pH (pH 5), cationic BS removed Cr(III) by 98%. The positive charges acquired by the cationic BS were due to the overexpression of the abovementioned amino acids (15.63% as evidenced from the composition of amino acids, Table 3), which may explain the enhanced removal of Cr(III) from the aqueous solution. At the optimized pH (pH 5), cationic BS is protonated, as evidenced from FT-IR spectroscopy of histidine. The peak at 3082.6 cm−1 could be attributed to the presence of symmetric N–H stretching and protonated NH2+ group present in histidine.44 The in-plane bending of C–H (ring) at 1121 cm−1 and 1156 cm−1 corresponds to the cationic form of phenylalanine.45The protonated amino acids (histidine, phenylalanine and tyrosine) at pH 5, which are lower than their isoelectric points release protons due to the delocalization of electrons caused by resonance (as evidenced from UV-Vis spectroscopy, Fig. 9a). The presence of O− after deprotonation is confirmed from the stretching vibration at 1591.23 cm−1.46
The non-bonded electrons at nitrogen of histidine, tyrosine and phenyl alanine stabilize the bonding with Cr(III) through coordinate linkage (as evidenced from the shift in frequency, as recorded by FT-IR spectroscopy).
After the interaction of cationic BS with Cr(III) ions, the peaks such as 3443.35 cm−1 and 3082.6 cm−1 are broadened and also the O− vibration peak disappeared. The in-plane C–H bending vibration corresponding to phenylalanine disappeared. This clearly suggests that Cr(III) binds with nitrogen of histidine, oxygen of tyrosine and nitrogen of phenyl alanine to form a co-ordinate bonding. The shift in the vibrational frequency, corresponding to N–H stretching of the peptide from 3297.26 to 3139 cm−1 and the shift in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the peptide group from 1658.41 cm−1 to 1651.41 cm−1, were observed after the interaction of Cr(III) with cationic BS. These vibrational shifts in the Cr(III) interacted cationic BS matrix is evidence for stabilization of bonding of Cr(III) with the respective amino acids. The schematic of the removal of Cr(III) by cationic BS is shown in Fig. 14.
O stretching of the peptide group from 1658.41 cm−1 to 1651.41 cm−1, were observed after the interaction of Cr(III) with cationic BS. These vibrational shifts in the Cr(III) interacted cationic BS matrix is evidence for stabilization of bonding of Cr(III) with the respective amino acids. The schematic of the removal of Cr(III) by cationic BS is shown in Fig. 14.
The anionic charge (−33.01 mV as evidenced from the zeta potential profile, Fig. 3) of BS could be due to the presence of sulphur-containing amino acids, cysteine, in lipoprotein. Cysteine has an isoelectric point (pI 5.02) much lower than the pH of BS. At the optimized pH (pH 6), the anionic BS acquires a negative charge due to the ionization of cysteine alone (2.85% as evidenced from composition of amino acid in anionic BS, Table 3). Under the optimized conditions, anionic BS ionically bonded with Cr(III). Cr(III) has possible bonding only with cysteine of anionic BS, which is poorly expressed in anionic BS and thus accounting for the poor removal (85%) of Cr(III) by the anionic surfactant.
The charged centre of cysteine coordinated with Cr(III) as evidenced from the change in frequency, as recorded by FT-IR spectroscopy. The FT-IR spectrum of anionic BS showed a band at 1560 cm−1 corresponding to the N H bend in cysteine.47 In addition, a weak broadened band at 2511 cm−1 may be due to the S–H stretching in cysteine molecule. After the interaction of anionic BS with Cr(III), the shift in the N–H bending vibration to 1552 cm−1 was observed. In addition, the disappearance of weak S–H stretching band clearly indicates that Cr(III) binds with the sulphur containing amino acid through ionic bonding. The vibrational shift in the peak corresponding to N–H stretching of the peptide group from 3451 to 3395 cm−1 was evident. The shift in C–N stretching vibration from 725 cm−1 to 713 cm−1 after ionic bonding of anionic BS with Cr(III) was observed. These vibrational shifts suggest that alteration in the peptide linkage as a consequence of the interaction of Cr(III) with anionic BS.
The anionic BS containing cysteine (Table 3) alone is responsible for ionic bonding with the sulphur group present in BS. Therefore, Cr(III) was removed only by 85% with anionic BS, whereas cationic BS removed Cr(III) by 98% at the same concentration (2.5 g). The mechanism for the removal of Cr(III) by anionic BS is illustrated in Fig. 15.
It is evident from the EDX spectra that anionic BS-Cr(III) contained Cr(III) by 0.18% of ions (Fig. 13a) and the cationic BS-Cr(III) contained Cr(III) by 0.44% (Fig. 13b). The fluorescence spectra also confirmed the removal of Cr(III) by cationic and anionic BS (Fig. 10).
4. Conclusions
In the present investigation, the production of anionic and cationic BS using palm oil and coconut oil as substrates, respectively, and their ability to remove Cr(III) ions from aqueous solutions were studied. The agar double diffusion technique and zeta potential measurements of biosurfactants confirmed the charge carried by the surfactants. The composition of the biosurfactants confirmed that they belong to the lipoprotein type. The biosequestration of Cr(III) ions by biosurfactants was confirmed by FT-IR analysis through their shifts in the peptide group of BS. The cationic biosurfactant showed that the maximum removal of Cr(III) ions from an aqueous solution was 98% at a biosurfactant concentration of 2.5 g through coordinate bonding, whereas the anionic biosurfactant removed 85% Cr(III) at the same concentration of biosurfactant through ionic bonding. To the best of our knowledge, this is the first report of interaction of cationic BS with Cr(III) ions in an aqueous solution.Acknowledgements
P. Saranya is thankful to the Council of Scientific and Industrial Research (CSIR), India. The financial assistance under the STRAIT (CSC0201) programme is also highly acknowledged.References
- J. H. Sharphouse, Leather Technician’s Handbook, Leather Producers Association, Buckland Press Ltd, London, 1989 Search PubMed.
- B. Basaran, M. Ulaş, O. Behzat and A. Band Aslan, Indian J. Chem. Technol., 2008, 15, 511–514 CAS.
- Environment Commission of I.U.L.T.C.S., IUE Recommendations on Cleaner Technologies for Leather Production, London, 1997.
- Central Pollution Control Board, The Environment (Protection) Rules, India, 1986, p. 418 Search PubMed.
- Z. X. Wang, J. Q. Chen, L. Y. Chai, Z. H. Yang, S. H. Huang and Y. Zheng, J. Hazard. Mater., 2011, 190, 980–985 CrossRef CAS PubMed.
- L. Di Palma, D. Mancini and E. Petrucci, Chem. Eng. Trans., 2012, 28, 145–150 Search PubMed.
- D. C. Sharma, C. Chatterjee and C. P. Sharma, J. Exp. Bot., 1995, 25, 241–251 Search PubMed.
- H. M. Abdulla, E. M. Kamal, A. H. Mohammed, and A. D. EI Bassuony, Proc. fifth Scien. Environ conf. Zagazig uni, 2010, pp. 171–183 Search PubMed.
- J. Kotas and Z. Stasicka, Environ. Pollut., 2000, 107, 263–283 CrossRef CAS.
- Ecological Analysts, Inc, The sources, chemistry, fate and effects of Chromium in aquatic environments, American Petroleum Institute, 2101 L St., N.W., Washington, DC 20037, 1981, p. 207 Search PubMed.
- M. M. Altaf, F. Masood and A. Malik, Turk. J. Biol., 2008, 32, 1–8 CAS.
- M. P. Plkociniczak, G. A. Płaza, Z. P. Seget and S. S. Cameotra, Int. J. Mol. Sci., 2011, 12, 633–654 CrossRef PubMed.
- P. Das, S. Mukherjee and R. Sen, Bioresour. Technol., 2009, 100, 4887–4890 CrossRef CAS PubMed.
- G. Dehghan Noudeh, M. Housaindokht and B. S. Bazzaz, J. Microbiol. Methods, 2005, 43, 272–276 Search PubMed.
- A. Franzetti, I. Gandolfi, G. Bestetti and I. M. Banat, Trends Biorem. Phytorem., 2010, 145–156 CAS.
- J. Thaniyavarn, A. Chongchin, N. Wanitsuksombut, S. Thaniyavarn, P. Pinphanichakarn, N. Leepipatpiboon, M. Morikawa and S. Kanaya, J. Gen. Appl. Microbiol., 2006, 52, 215–222 CrossRef CAS.
- V. Walter, C. Syldatk and R. Hausmann, Bioscience and Springer Science, Business Media, 2010 Search PubMed.
- R. D. Rufino, J. M. Luna, G. M. Campos-Takaki, S. R. M. Ferreira and L. A. Sarubbo, Chem. Eng. Trans., 2012, 27, 61–66 Search PubMed.
- T. Meylheuc, C. J. Vanoss and M. M. Bellon-Fontaine, J. Appl. Microbiol., 2001, 91, 832 CrossRef.
- D. G. Cooper and B. G. Goldenberg, Appl. Environ. Microbiol., 1987, 53, 224–229 CAS.
- T. Varadavenkatesan and V. R. Murty, J. Microbiol. Biotechnol. Res., 2013, 3(4), 63–73 CAS.
- M. Dubois, K. A. Gills, J. K. Hamilton, P. A. Rebers and F. Smith, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–275 CAS.
- J. Izard and R. J. Limberger, J. Microbiol. Methods, 2003, 55, 411–418 CrossRef CAS.
- S. Ramakrishnan, K. N. Sulochana, R. Punitham and K. Arunagiri, Glycoconjugate J., 1996, 13, 519–523 CrossRef CAS.
- U. K. Laemmli, Nature, 1970, 227, 680–685 CrossRef CAS PubMed.
- W. M. F. W. Nawawi, Optimum Biosurfactant production from sludge palm oil using Klebsiella pneumoniae, International Islamic University Malaysia, Kuala Lumpur, Malaysia, 2011 Search PubMed.
- S. Patil, A. Pendse and K. Aruna, Int. J. Curr. Biotechnol., 2014, 2, 20–30 CAS.
- M. Kannahi and M. Sherley, Int. J. Chem. Pharm. Sci., 2012, 3, 37–42 CAS.
- S. K. Satpute, B. D. Bhawsar, P. K. Dhakephalkar and B. A. Chopade, Indian J. Mar. Sci., 2008, 37, 243–250 CAS.
- K. Ramani, S. Chandan Jain, A. B. Mandal and G. Sekaran, Colloids Surf., B, 2012, 97, 254–263 CrossRef CAS PubMed.
- S. S. Bhadoriya, N. Madoriya, K. Shukla and M. S. Parihar, Biochem. Pharmacol., 2013, 2, 113 Search PubMed.
- S. H. Kim, E. J. Lim, S. O. Lee, J. D. Lee and T. H. Lee, Biotechnol. Appl. Biochem., 2000, 31, 249–253 CrossRef CAS.
- T. T. Nguyen, N. H. Youssef, M. J. McInerney and D. A. Sabatini, Water Res., 2008, 42, 1735–1743 CrossRef CAS PubMed.
- P. Saranya, S. Swarnalatha and G. Sekaran, RSC Adv., 2014, 4, 34144–34155 RSC.
- C. N. Mulligan and B. F. Gibbs, Proc. Natl. Acad. Sci. U. S. A., 2004, 1, 31–55 Search PubMed.
- G. Bognolo, Colloids Surf., A, 1999, 152, 41–52 CrossRef CAS.
- E. Z. Ron and E. Rosenberg, Appl. Microbiol. Biotechnol., 1999, 52, 154–162 CrossRef.
- A. A. Juwarkar, K. V. Dubey, A. Nair and S. Kumar Singh, Indian J. Microbiol., 2008, 32, 142–146 CrossRef PubMed.
- D. Kratochvil, P. Pimentel and B. Volesky, Environ. Sci. Technol., 1998, 32, 2693–2698 CrossRef CAS.
- A. Nasreen, I. Muhammad, Z. Saeed Iqbal and I. Javed, J. Environ. Sci., 2008, 20, 231–239 CrossRef.
- K. K. Singh, R. Rastogi and S. H. Hasan, J. Colloid Interface Sci., 2005, 290, 61–68 CrossRef CAS PubMed.
- P. Miretzky and A. Fernandez Cirelli, J. Hazard. Mater., 2010, 180, 1–19 CrossRef CAS PubMed.
- K. Rajarajan, K. Anbarasan, J. Samu Solomon and G. Madhurambal, J. Chem. Pharm. Res., 2012, 4, 4060–4065 CAS.
- S. Olsztynska, M. Komorowska, L. Vrielynck and N. Dupuy, Appl. Spectrosc., 2001, 55, 901–907 CrossRef CAS.
- A. Barth, Prog. Biophys. Mol. Biol., 2000, 74, 141–173 CrossRef CAS.
- S. Aryal, B. K. C. Remant, N. Dharmaraj, N. Bhattarai, C. H. Kim and H. Y. Kim, Spectrochim. Acta, Part A, 2006, 63, 160–163 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra07999c |
| This journal is © The Royal Society of Chemistry 2015 |