Solubilization of waste activated sludge using a garbage enzyme produced from different pre-consumer organic waste
C. Arun and
P. Sivashanmugam*
Department of Chemical Engineering, National Institute of Technology, Tiruchirappalli, 620015, Tamil Nadu, India. E-mail: psiva@nitt.edu; lrcarun@gmail.com; Fax: +91 431 2500133; Tel: +91 431 2503106
First published on 29th May 2015
Abstract
The conversion of pre-consumer solid waste into value added products, and utilisation of this for the treatment of activated sludge into a reusable form without having toxic effects on the environment, is much more in focus in the present day. In the present work, different types of garbage enzyme were produced from pre-consumer waste (pineapple, cauliflower, orange, tomato, and mango dregs) and the characteristics of each garbage enzyme produced were investigated. Sludge solubilization was performed with different types of garbage enzyme at different pH and time. When the treatment time increased from 48–60 hours, a higher reduction of VSS (Volatile Suspended Solids), TSS (Total Suspended Solids) and also a higher increase of the solubility of COD (Chemical Oxygen Demand), TKN (Total Kjeldhal Nitrogen) and TP (Total Phosphorus) were obtained for all types of garbage enzyme at pH 7. The pineapple and orange garbage enzyme showed a higher reduction % of VSS and TSS of nearly 20–25% and also increased % solubilization of COD, TKN and TP by nearly 20–25%, 15–20% and 9–11% respectively in treated WAS (Waste Activated Sludge) compared with other garbage enzymes. This significant result showed that a garbage enzyme solution has the capability to solubilize complex (insoluble organic) compounds to soluble organic compounds, which can be subsequently treated by anaerobic microbes to produce methane or hydrogen.
1. Introduction
In recent decades the development of food processing industries is an increasing trend in developing countries. These types of industries are producing pre-consumer vegetable and fruit organic waste. On the one hand improper disposal of these organic wastes along with other municipal solid waste in open dumps, generates unpleasant odours and increases disease-causing organisms affecting human health.1 On the other hand organic waste disposal by landfill methods produce greenhouse gases and leachate affecting the atmosphere and the water environment to a larger extent.2 The organic waste and sludge in landfill will ultimately degrade to produce carbon dioxide and methane thereby recirculating carbon back to the atmosphere and causing global warming.3 The discharge of greenhouse gases (GHGs) into the atmosphere is expected to have significant impact on the environment, human health and the economy. Subsequently environment-friendly and sustainable technology at a low cost is needed for the management and reuse of pre-consumer organic waste.4 The pre-consumer organic waste can be used to produce garbage enzymes by fermentation. Garbage enzymes can be used as fertilizer, plant growth hormone, pesticides, insecticides, wastewater treatments and antimicrobial agents.5The number of wastewater treatment plants, for industrial and domestic (municipal) wastewater, is increasing day by day to achieve the permissible limit for discharge of wastewater stipulated by environmental conservation and protection organisations like the WHO (World Health Organization), pollution control boards etc. Due to the increase of wastewater treatment plants, the generation of sludge from them has also increased significantly. The sludge produced is usually rich in poorly stabilised organic matter, affecting the air, water and soil environments during storage and land spreading. The management of the high amounts of sludge generated has become one of the challenging tasks for wastewater treatment plants.6 Incineration and landfilling are the most common methods used to dispose of sludge from wastewater treatment plants. Recent legislation in developing countries is forcing industries to reduce the amount of sludge entering landfills and adopt alternate methods to increase the recycling of sludge. Anaerobic digestion and composting are suitable technologies to treat the solid waste and have been considered as waste to wealth technology.7,8 The operating cost of treatment of high-organic industrial wastewater is less by anaerobic digestion than by aerobic composting.9 The production of biogas through anaerobic digestion offers the most environment-friendly and energy-efficient technology for bioenergy production. The anaerobic digestion process has four essential stages namely hydrolysis, acidogenesis, acetogenesis and methanogenesis. Among these stages, the hydrolysis stage is a rate-limiting step10 as it involves depolymerisation of complex organic matter (insoluble state). This problem can be overcome by solubilizing the insoluble complex organic matter before entering anaerobic digestion, because when the organic matter is in the soluble state, the microorganisms can digest the organic matter at a faster rate without further breakdown. Various physical,11,12 chemical,13–15 and biological methods16–19 are available to solubilize the complex organic matter, but the biological (microbial or enzyme) methods are preferred due to being eco-friendly and having a low operating cost.20,21 In addition, these methods are preferred to improve the solubility of sludge for further utilization or disposal. In enzymatic hydrolysis, enzymes act on the WAS and release nutrients in a soluble form with a reduction of solids.22 Guo and Xu23 reported that mostly in the biological treatment, the hydrolysis and degradation of complex biodegradable organic matter depended on the presence of hydrolytic enzymes. Nagina et al.24 reported that the alkaline protease, a hydrolytic enzyme, showed a beneficial effect in pathogen reduction, solid reduction and also improved dewatering of sewage sludge. Roman et al.25 investigated the combined effect of commercially available enzymes (cellulase and pronase E) in solubilizing organic municipal waste activated sludge. All of the above cited investigations were based on the hydrolysis of municipal sludge treated with commercial enzymes. Fazna and Meera26 studied the treatment of grey water using 5% and 10% of garbage enzyme and confirmed that 10% garbage enzyme has the ability to reduce BOD, COD and TDS by up to 70, 50, and 39% respectively. Tang and Tong27 reported that a 9% solution of garbage enzyme in wastewater was found to be the most cost-effective in removing ammonia nitrogen and phosphorus, and also neutralizing the domestic wastewater. Till now no attempt has been made to solubilize industrial waste activated sludge using garbage enzymes. Also, the garbage enzyme production cost is cheaper as it is produced from organic solid waste and hence one can get the advantage of both solid waste treatment of pre-consumer organic waste and activated sludge solubilization.
Therefore in the present work, an attempt was made to produce different types of garbage enzyme from pre-consumer waste (pineapple, cauliflower, orange, tomato, and mango dregs separately) and the characteristics of each garbage enzyme produced were investigated. Also, experiments were performed for the solubilization of dairy waste activated sludge using different crude garbage enzymes. Parameters like VSS, TSS, soluble COD, soluble total Kjeldhal nitrogen, and soluble total phosphorus before and after treatment were studied to find out the effect of treatment time and pH on the solubilization of WAS.
2. Materials and methods
2.1 Production of garbage enzyme from different types of pre-consumer organic waste
In this study pre-consumer organic waste like pineapple, orange, tomato, cauliflower, and mango peel and dregs were collected from vegetable markets and fruit shops in Tiruchirappalli and stored in a refrigerator at 4 °C for the production of garbage enzymes. Five 2 liter airtight containers were taken and named as PGE (pineapple garbage enzyme), OGE (orange garbage enzyme), TGE (tomato garbage enzyme), CGE (cauliflower garbage enzyme), and MGE (mango garbage enzyme). To each container 500 mL of water and 50 grams of molasses were added with sufficient mixing. 150 grams of pineapple peel was added and well mixed in the PGE container and this procedure was repeated for the remaining four containers with the respective pre-consumer waste. These airtight containers were placed in a cool, dry and well-ventilated area for three months of fermentation.2.2 Characterization of different types of garbage enzyme
After three months of fermentation, the solution from each container was filtered and centrifuged at 3000 rpm for 30 minutes and the purified solutions were stored separately in a refrigerator at 4 °C. Parameters like pH, TS (total solids), TDS (total dissolved solids), BOD (biological oxygen demand), COD and MPN (most probable number) of the different types of garbage enzyme were analysed according to the standard methods.28 Citric acid concentration was determined using HPLC method and is presented in Table 1. From Table 1 it is observed that all the above analysed parameters are more or less equal in all of the enzyme solutions, and these values are taken into account while determining the environmental parameters of treated WAS with garbage enzyme solution.| Parameters | PGE | OGE | TGE | CGE | MGE |
|---|---|---|---|---|---|
| pH | 3.4–3.7 | 3.2–3.3 | 3.1–3.4 | 3.4–3.6 | 3.5–3.7 |
| TDS (mg L−1) | 997–1006 | 995–1008 | 1013–1019 | 1006–1020 | 1009–1027 |
| BOD (mg L−1) | 70–79 | 65–74 | 69–81 | 67–79 | 71–78 |
| COD (mg L−1) | 150–157 | 152–160 | 151–158 | 154–160 | 151–154 |
| MPN (C.F.U mL−1) | <3 | <3 | <3 | <3 | <3 |
| Citric acid (mg mL−1) | 2.367 | 4.402 | 1.483 | 1.075 | 0.5734 |
Cell-free enzyme activities of the garbage enzymes were determined by centrifuging 10 mL of solution at 3000 rpm for 10 min. The supernatant was collected and used for the measurement of cell-free enzyme activity. Amylase activity was measured using the method of Bernfeld.29 The assay solution containing 0.5 mL of 1.0% soluble starch solution and 0.5 mL of enzyme solution was incubated at 25 °C for 10 min and 1 mL of dinitrosalicylic acid colour reagent was added. Then the mixture solution was incubated in a boiling water bath for 5 minutes and cooled to room temperature. The absorbance of the mixture was read at 540 nm. The reducing groups, namely maltose, released from starch were measured by the reduction of 3,5-dinitrosalicylic acid.
1 mL of garbage enzyme solution was mixed with 1 mL of 2% casein and the resulting solution was pre-warmed for 10 min to allow the reaction to proceed. The reaction was then terminated by the addition of 2 mL of trichloroacetic acid solution and then incubated in a water bath at 35 °C for 10 min. After centrifugation of this mixture at 3000 rpm, 1 mL of supernatant was taken and to it 5 mL of Na2CO3 and 1 mL of folin phenol reagent were added.30 The absorbance of the mixture was read at 660 nm. The activity of protease was expressed as the amount of enzyme that releases 1 mg of tyrosine equivalent per minute.
Lipase activity was determined spectrophotometrically using the procedure of Pandey31 et al. The reaction mixture contained 50 μl of enzyme solution and 950 μl of substrate solution (1 part of 3.0 mM p-NPPin 2 propanol with 9 parts of 0.4% Triton X100 and 0.1% gum Arabic). The reaction mixture was incubated at 37 °C for 20 min and the absorbance of the mixture was read at 410 nm. The activity of lipase was expressed as the amount of enzyme that releases 1 μmole of p-nitrophenol per minute of tyrosine equivalent per minute.
2.3 Sampling and characterization of WAS sludge
The waste activated sludge (WAS) was collected from a dairy at Trichy in Tamil Nadu (India) and stored in a refrigerator at 4 °C. The characteristics of the raw sludge namely pH, TS, VSS, TSS, BOD, TCOD (total chemical oxygen demand), SCOD (soluble chemical oxygen demand), TKN (Total Kjeldhal Nitrogen), STKN (Soluble Total Kjeldhal Nitrogen), TP (total phosphorus), and STP (soluble total phosphorus) were analysed according to APHA methods.28 Total protein in the sludge was analysed with the help of Lowry’s method, carbohydrates were analysed by the phenol sulphuric acid method and the results are presented in Table 2.| Parameters | Value |
|---|---|
| pH | 6.7–7.2 |
| Total solids | 9038 mg L−1 |
| Volatile suspended solids | 4971 mg L−1 |
| Total suspended solids | 5034 mg L−1 |
| Total COD | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 094 mg L−1 094 mg L−1 |
| Soluble COD | 853 mg L−1 |
| TKN | 1209 mg L−1 |
| STKN | 283 mg L−1 |
| TP | 326 mg L−1 |
| STP | 25 mg L−1 |
| Total protein | 814 mg L−1 |
| Carbohydrates | 366 mg L−1 |
| MPN (C.F.U per 100 mL) | 9.7 × 107 |
2.4 Treatment of sludge using different types of garbage enzyme
20 mL of the concentrated PGE, OGE, TGE, CGE and MGE enzyme solutions was diluted with 200 mL of ultra-pure water. The pH of the garbage enzyme was adjusted to 3.5 and 7 with the help of sodium citrate and phosphate buffer solution. These diluted garbage enzyme solutions with adjusted pH were used for the treatment to improve the solubilization of COD, TKN and TP in WAS. Five 250 mL conical flasks were taken and 20 grams of WAS was added in all the flasks. After this 50 mL of diluted PGE, OGE, TGE, CGE and MGE enzyme were added separately in all the flasks, labelled respectively. Another 250 mL conical flask labelled as control was taken and 20 grams of WAS only was added with the respective buffer solution. All the conical flasks were kept in an incubator shaker at 100 rpm and sludge treatment experiments were conducted for 60 hours by maintaining the temperature at 35 °C. The solubility of the sludge was evaluated by determining the COD solubilization, VSS and TSS reduction and nutrient (nitrogen and phosphorus) solubilization after treatment. At regular time intervals the above parameters were estimated and the experiments were repeated twice to determine the consistency in the results obtained. The increases in COD solubilization %, STKN % and STP % were calculated by following eqn (1)–(3) respectively.
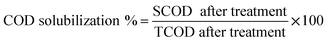 | (1) |
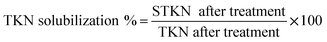 | (2) |
 | (3) |
3. Results and discussion
3.1 Hydrolytic enzyme activity in garbage enzyme solutions
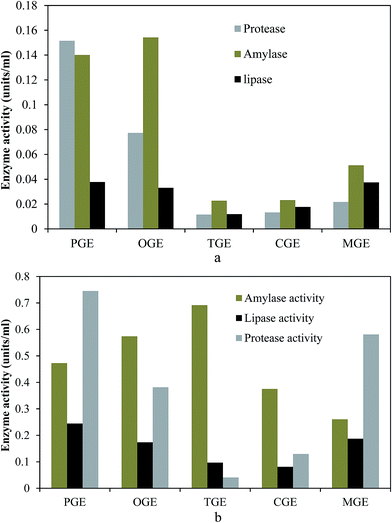
In the present study cell-free hydrolytic enzyme activities in garbage enzyme solutions produced from different pre-consumer organic waste were determined and the results are presented in Fig. 1a and b. From these figures, it is observed that all types of garbage enzyme at pH 3.5 and pH 7 have amylase, protease and lipase activity. Hydrolytic enzyme activity is higher for garbage enzyme solutions with pH 7 when compared to garbage enzyme solutions with pH 3.5. Among them the amylase activity is higher for the tomato garbage enzyme solution and lower for mango garbage enzyme. Similarly protease activity is higher for the pineapple garbage enzyme solution and lower for the tomato garbage enzyme solution. Lipase activity is higher for the pineapple garbage enzyme and all other garbage enzyme solutions possess comparable lipase activity. Thus this experiment confirms the presence of hydrolytic enzyme activity in all types of garbage enzyme solution at pH 7 is higher when compared with pH 3.5.3.2 VSS and TSS reduction
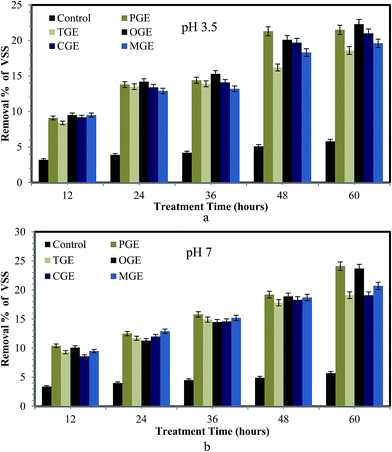
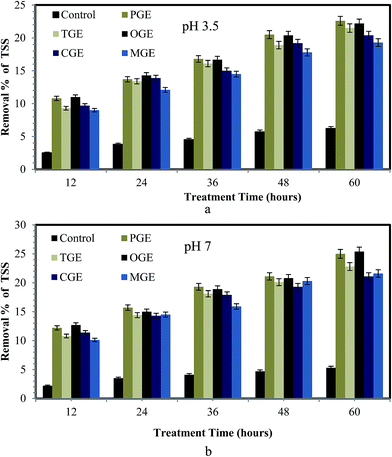
The stability and effectiveness of the sludge treatment process can be determined using VSS and TSS reduction.32 The removal percentages of volatile solids and suspended solids from sludge after treatment with different types of garbage enzyme (pH 3.5 and 7) are presented in Fig. 2a and b and 3a and b respectively. From these figures it is observed that the removal percentage of VSS and TSS increased for all types of garbage enzyme, when the treatment time increased from 12–60 hours at both values of pH. But significantly, a higher reduction in VSS and TSS is observed for the sludge treated with garbage enzyme at pH 7 when compared with garbage enzyme at pH 3.5. The reason for the higher reduction of VSS and TSS at pH 7 is due to enhanced activity of hydrolytic enzymes at pH 7 whereas enzyme activity was suppressed at pH 3.5 due to the acidic conditions. Similarly Qi Yanga et al.,22 demonstrated municipal secondary sludge treatment with protease, amylase, and mixed-enzyme treatment and concluded that the solid reduction was found to be 42%, 56.32% and 68.43% respectively.It is also observed that WAS treated with PGE and OGE showed an increase in VSS and TSS reduction of 21–25%. The reason for higher VSS and TSS reduction by PGE and OGE treated sludge is explained as follows. OGE contains organic acids, mainly citric acid, as it was produced from the fermentation of citrus fruit peels. Citric acid has the power to disturb the extracellular polymeric substances (EPS) and release hydrolytic enzymes.12,33 In addition to garbage enzyme these released hydrolytic enzymes also have an impact on sludge solubilization. Thus citric acid has the ability to enhance the sludge matrix breakage, which in turn resulted in higher VSS and TSS reduction %, when sludge was treated with OGE. MGE has a lower citric acid concentration when compared to other garbage enzyme thus it shows lower removal % of solids (Table 1).
The PGE solution is produced by fermentation of the peel of pineapple along with water and molasses. During the production of this enzyme, at acidic conditions protease from the peel of the pineapple was released into the garbage solution. This extracellular proteolytic enzyme has a higher activity at pH 7, which activates the hydrolysis of protein present in dairy waste activated sludge. Because of this reason the VSS and TSS reduction % is increased when sludge is treated with PGE.
3.3 COD solubilization
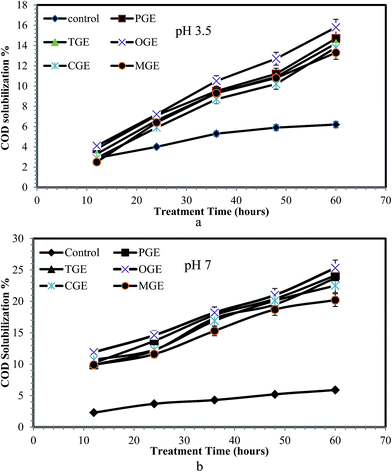
The treatment process of sludge aims to improve the biodegradability and bioavailability of sludge organic matter in a soluble form. The increase in biodegradability is directly proportional to the solubilized COD.34,35 The SCOD calculation is considered as a main parameter for the evaluation of the maximum level of sludge solubilization.32 Fig. 4a and b, present the effect of different garbage enzymes on COD solubilization of WAS at pH 3.5 and 7 respectively. From Fig. 4a and b, it is observed that the COD solubilization of WAS at both pHs (3.5 and 7) starts increasing for all types of garbage enzyme (PGE, OGE, TGE, CGE, MGE) when compared to the control (WAS with the respective buffer solution) while the treatment time increased from 12–60 hours. Also, the sludge treated with garbage enzymes at pH 7 showed a significant increase in COD solubilization, compared with garbage enzymes at pH 3.5. The reason for a higher COD solubilization rate at pH 7 is due to the enhanced activity of hydrolytic enzymes at that pH whereas its activity got suppressed at pH 3.5 (acidic), due to a loss in enzyme stability. The increase in SCOD level in treated sludge indicates that the sludge contains a large amount of soluble substances. When organic particles are solubilized it can be readily degraded by microorganisms during anaerobic digestion processing to produce biogas. Similarly Roman et al.25 investigated the combined effect of commercially available enzymes (cellulase and pronase E) in solubilizing organic municipal waste activated sludge (MWAS) and reported the increases in SCOD level in MWAS after treatment with the enzymes.3.4 TKN and TP solubilization
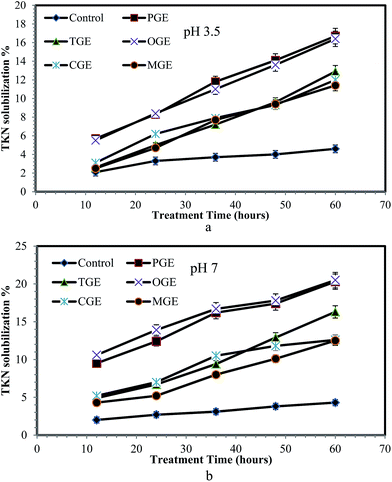
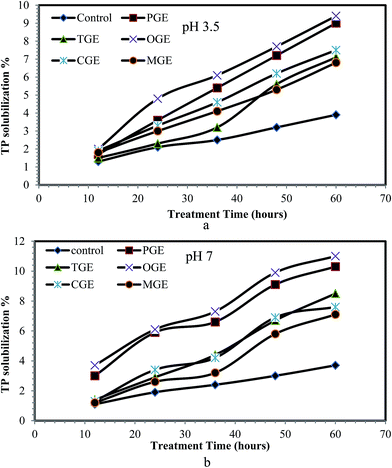
WAS contains a large amount of nitrogenous compounds in the form of organic nitrogen, ammonia, and ammonium and most of them are in insoluble complex form, namely amino acids, amino sugars and proteins.36 By observing the characteristics of WAS before treatment with garbage enzyme solution (Table 2) it is seen that less than 20–25% of nitrogenous compounds are in a soluble form and the remaining 75–80% are insoluble in nature. Therefore solubilization processing of such waste activated sludge is required to increase the soluble nitrogen components, which in turn minimizes the rate-limiting hydrolysis stage during biological treatment of sludge. Hence, the sludge was treated with different garbage enzyme solutions and the STKN content in WAS after treatment with respect to treatment time is presented in Fig. 5a and b. From Fig. 5a and b, it is observed that soluble TKN increases when compared to the control while the treatment time increases from 12 to 60 hours. The reason for the increasing soluble TKN % is due to the presence of organic acids (carbon source) in garbage enzyme solutions.By observing the characteristics of WAS before treatment with garbage enzyme solution (Table 2) it is seen that less than 9% of phosphorus is in a soluble form and the remaining 91% is in an insoluble form. The phosphorus content of waste activated sludge includes orthophosphate, polyphosphate and organic phosphate. Polyphosphate (insoluble) in sludge should be converted to orthophosphate (soluble) by the process of hydrolysis.36 Therefore, WAS was treated with different garbage enzyme solutions and the STP in WAS after treatment with respect to treatment time is presented in Fig. 6a and b. From Fig. 6a and b, it is observed that the soluble phosphorus in WAS increases when compared to the control while the treatment time increases from 12 to 60 hours for all types of garbage enzyme. The maximum increases of STKN (15–20%) and STP (9–11%) were found when the sludge was treated with PGE and OGE.
The reason for the increase in solubilization of TKN and TP in treated sludge is due to the presence of organic acid (carbon source) and hydrolytic enzyme in the garbage enzyme solution, which helped in the breakdown of the insoluble form of minerals to the soluble from. Ely Nahas37 reported a similar observation, when investigating the microbial solubilization of phosphorus, carbon and nitrogen in soil.
4. Conclusion
The cell-free hydrolytic enzyme activities in garbage enzyme solutions produced from different pre-consumer organic waste were determined. Thus these experiments confirm the presence of hydrolytic enzyme activity in all types of garbage enzyme solution at pH 7. The WAS treatment was performed with different types of garbage enzyme at pH 3.5 and 7 and different treatment times (12, 24, 36, 48 and 60 hours). The pineapple and orange garbage enzymes showed a slightly higher reduction % of VS and SS of nearly 20–25%, and an increased % solubilization of COD, TKN and TP of nearly 20–25%, 15–20% and 9–11% respectively in treated WAS. The above significant results showed that garbage enzyme solutions have the capability to solubilize complex insoluble organic compounds to soluble organic compounds, which can be subsequently treated by anaerobic microbes to produce methane or hydrogen.References
- D. Hoornweg and P. Bhada, What a waste-A Global Review of Solid Waste Management, Urban Development Series, World Bank, 15, Washington, USA, 2012 Search PubMed.
- D. Pleissner and C. Sze Ki Lin, Sustainable Chem. Processes, 2013, 1, 21 CrossRef.
- E. Levlin, International scientific seminar, Research and application of new technologies in wastewater treatment and municipal solid waste diposal in Ukraine, Sweden and Poland 23-25, September 2009 Stockholm, Polish-Swedish, TRITALWR report, 2010, 3026, pp. 95–104 Search PubMed.
- R. Luque and J. H. Clark, Sustainable Chem. Processes, 2013, 1, 10 CrossRef.
- B. Prakash, 2011, http://www.ecowalkthetalk.com, accessed 17th December 2013.
- E. Neyens and J. Baeyens, J. Hazard. Mater., 2003, 98, 51–67 CrossRef CAS.
- V. Sonakya, N. Raizada and V. C. Kalia, Biotechnol. Lett., 2001, 23, 1463–1466 CrossRef CAS.
- V. K. Garg and G. Renuka, Ecotoxicol. Environ. Saf., 2011, 74, 19–24 CrossRef CAS PubMed.
- M. Clisso, 2010, http://water.me.vccs.edu/courses/env108/anaerobic.htm, accessed 17th November 2013.
- C. Bougrier, J. P. Delgenès and H. Carrère, Biochem. Eng. J., 2008, 34, 20–27 CrossRef PubMed.
- J. Xu, H. Yuan, J. Lin and W. Yuan, J. Taiwan Inst. Chem. Eng., 2014, 45, 2531–2536 CrossRef CAS PubMed.
- T. Gayathri, S. Kavitha, S. Adish Kumar, S. Kaliappan, I. Tae Yeom and J. Rajesh Banu, Ultrason. Sonochem., 2015, 22, 333–340 CrossRef CAS PubMed.
- T.-H. Kim, T.-H. Kim, S. Yu, Y. Ku Nam, D.-K. Choi, S. Ryul Lee and M.-J. Lee, J. Ind. Eng. Chem., 2007, 13(7), 1149–1153 CAS.
- N. H. Heo, S. C. Park, J. S. Lee and H. Kang, Water Sci. Technol., 2003, 48(8), 211–219 CAS.
- X. Zhou, G. Jiang, Q. Wang and Z. Yuan, RSC Adv., 2014, 4, 50644 RSC.
- M. Veera Lakshmi, J. Merrylin, S. Kavitha, S. Adish Kumar, J. Rajesh Banu and I.-T. Yeom, Environ. Sci. Pollut. Res., 2014, 21, 2733–2743 CrossRef PubMed.
- S. Kavitha, C. Jayashree, S. Adish Kumar, I.-T. Yeom and B. J. Rajesh, Bioresour. Technol., 2014, 168, 159–166 CrossRef CAS PubMed.
- J. Merrylin, S. Adish Kumar, S. Kaliappan, I.-T. Yeom and B. J. Rajesh, Environ. Technol., 2013, 34, 2113–2123 CrossRef CAS PubMed.
- S. Kavitha, T. Saranya, S. Kaliappan, S. A. Kumar, I. Tae and J. R. Banu, Bioresour.Technol., 2015, 175, 396–405 CrossRef CAS PubMed.
- T. Imai, Y. Liu, M. Ukita and Y.-T. Hung, Environmental Bioengineering, Handbook of Environmental Engineering, 2010, 11, pp. 75–122 Search PubMed.
- W. Parawira, Crit. Rev. Biotechnol., 2012, 32, 172–186 CrossRef CAS PubMed.
- Q. Yanga, K. Luo, X.-m. Li, D.-b. Wang, W. Zheng, G.-m. Zeng and J.-j. Liu, Bioresour. Technol., 2010, 101, 2924–2930 CrossRef PubMed.
- J. S. Guo and Y. F. Xu, Review of Enzymatic Sludge Hydrolysis, J. Biorem. Biodegrad., 2011, 2, 130 Search PubMed.
- N. Parmar, A. Singh and P. W. Owen, World J. Microbiol. Biotechnol., 2001, 17, 169–172 CrossRef CAS.
- H. J. Roman, J. E. Burgess and B. Pletschke, Afr. J. Biotechnol., 2006, 5(10), 963–967 CAS.
- F. Nazim and V. Meera, Bonfring International Journal of Industrial Engineering and Management Science, 2013, 3(4), 111–117 CrossRef.
- F. E. Tang and C. W. Tong, World Academy of Science, Engineering and Technology, 2011, 60 Search PubMed.
- American public Health Association (APHA), Standard Methods for examination of water and waste water, Washington, DC, 21st edn, 2005 Search PubMed.
- P. Bernfeld, Enzymes of starch degradation and synthesis, in Advances in Enzymology, ed. F. F. Nord, Interscience Publication, New York, 1951, p. 379 Search PubMed.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265 CAS.
- A. Pandey, S. Benjamin, P. Nigam, C. R. Soccol and N. Krieger, Biotechnol. Appl. Biochem., 1999, 29, 119–131 CAS.
- R. Uma Rani, S. Adish Kumar, S. Kaliappan, I.-T. Yeom and J. Rajesh Banu, Waste Manag., 2013, 33, 1119–1127 CrossRef CAS PubMed.
- J. Wawrzyńczyk, M. Recktenwald, O. Norrlöw and E. Dey Szwajcer, Afr. J. Biotechnol., 2007, 4(17), 1994–1999 Search PubMed.
- J. Dwyer, D. Starrenburg, S. Tait, K. Barr, D. J. Batstone and P. Lant, Water Res., 2008, 42, 4699–4709 CrossRef CAS PubMed.
- S. Kavitha, S. A. Kumar, S. Kaliappan, I. Tae and J. R. Banu, Bioresour. Technol., 2014, 169, 700–706 CrossRef CAS PubMed.
- G. Tchobanoglous, F. L. Burton, H. D. Stensel, Wastewater Engineering: Treatment and Reuse, McGraw-Hill, New York, 4th edn, 2002 Search PubMed.
- E. Nahas, First International Meeting on Microbial Phosphate Solubilization, Developments in Plant and Soil Sciences, 2007, 102, pp. 111–115 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |