Assembly of 3-allyl-3-ethynyl-oxindole motifs via palladium(II)-catalyzed quaternary allylation of 3-ethynyl-3-OBoc-oxindoles†
Nandarapu
Kumarswamyreddy
,
Muthuraj
Prakash
,
Samydurai
Jayakumar
and
Venkitasamy
Kesavan
*
Chemical Biology Laboratory, Department of Biotechnology, Bhupat and Jyoti Mehta School of Biosciences, Indian Institute of Technology Madras, Chennai-600036, India. E-mail: vkesavan@iitm.ac.in; Fax: +91-44-22574102; Tel: +91-44-2257-4124
First published on 15th June 2015
Abstract
Palladium(II)-catalyzed C3-allylation of 3-ethynyl-3-OBoc oxindole derivatives was achieved for the first time to access highly functionalized 3-allyl-3-ethynyl substituted oxindole derivatives for a broad range of substrates in very good yields (up to 94%). The synthetic applications of the allylated products were explored by synthesizing five and six membered carbocyclic spirooxindoles in moderate to very good diastereoselectivities.
Introduction
The development of new synthetic strategies to access 3,3-disubstituted oxindoles remains an important task in synthetic chemistry, because of their exciting and unique applications in biology.1 Despite the advances in organic synthesis to synthesize these motifs with diverse substitutions like N, O, S, etc.,2 only a few protocols are available to construct 3,3-dicarbon substituted oxindoles.3 Among various known 3,3-disubstituted oxindoles, introduction of allyl substitution at C3-position gained much attention, since they can eventually be exploited to synthesize various natural products and pharmaceutical lead compounds.4 In this regard, few racemic and chiral strategies have been reported in the literature to access 3-allyl substituted oxindoles. These include arylation of amides I,5 asymmetric allylic alkylation using oxindole prochiral nucleophiles6II and allylation of 3-hydroxy-oxindoles bearing electron rich aromatic ring systems III (Scheme 1).7 Despite these developments, to our surprise there is no literature precedence to construct 3-allyl-3-ethynyl substituted oxindoles 5. Furthermore, grafting allyl and ethynyl substitutions at C3-position of oxindole is highly desirable, since, ethynyl and allyl (1,5-enyne) combination is well exploited in the literature to build cyclic and complex structures.8 We hope that, incorporating ethynyl and allyl substitutions at C3-position of oxindole would enable the structural diversification around C3-position of oxindole, which is much desired in medicinal chemistry. Apart from its synthetic utility the presence of alkyne moiety at 3-position of oxindole showed higher anti-retroviral inhibition in HIV-1 than efavirenz.9 Hence, the identification of suitable combination of electrophile and nucleophile to access 3-allyl-3-ethynyl substituted oxindoles is of great importance.Various synthetic efforts were reported in the literature for the direct addition of allyltrimethylsilane to secondary propargyl alcohols Va using Lewis10a–j or Brønsted10k,l acid catalysts (Scheme 1). Despite, these accomplishments in the literature, only a very few protocols are available for the allylation of tertiary propargyl alcohols Vb or their derivatives.11 Moreover, there is no literature precedence on the allylic substitution of propargyl alcohol derivative in oxindole scaffold 2. Therefore, development of a general, efficient, catalytic method for propargylic substitution reaction comprising oxindole moiety is highly warranted.
Results and discussion
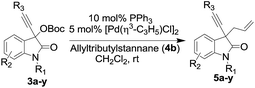
Our attempt began with the synthesis of 3-hydroxy-3-ethynylindolin-2-ones 2 by treating isatins 1 with alkynyl magnesium bromide generated in situ from corresponding alkyne using Grignard reaction in the absence of silver salt and pyrophoric reagent.9,12 This is the first report which discloses the synthesis of 3-hydroxy-3-ethynylindolin-2-ones 2a–y using in situ generated Grignard reagent (see ESI†).Initially, we attempted the direct allylation of 3-hydroxy-1-methyl-3-(phenylethynyl)indolin-2-one 2a with allyltrimethylsilane/allyltributylstannane 4a/4b in the presence of either Lewis acids or Brønsted acid in dichloromethane. In contrast to previous successful reports,10 allylation failed to occur in the presence of Lewis acid/Brønsted acid. Similar results were observed when trifluoroacetic acid and BF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et2O were employed as catalysts and the allylation of 2a did not occur even at elevated temperature under these conditions (see ESI†). It is reasonable to anticipate that, creation of cation at pseudobenzylic position in the absence of electron rich aromatic system is less favorable. Hence, we employed corresponding 3-OBoc oxindole derivative 3a as the reactant. However, the reaction of trimethylsilane 4a with 3-OBoc oxindole derivative 3a afforded only negligible amount of allylated product 5a under Lewis acid conditions (Table 1, entries 1–6). The allylation reaction between allyltributylstannane 4b and 3-OBoc oxindole derivative 3a afforded the desired product 5a in 38% yield when scandium triflate was employed as a catalyst (Table 1, entry 7). Screening of other Lewis acids under identical conditions did not bring in desired results (entries 8–12). Hence, we turned our attention to palladium catalysts to achieve the allylation at pseudobenzylic position due to their demonstrated potential in C–C bond formation.13 As a preliminary investigation, allylation reaction between allyltributylstannane 4b and 3-OBoc oxindole derivative 3a was carried out in the presence of 5 mol% of palladium(II) chloride and 10 mol% of PPh3 in dichloromethane at ambient temperature. The reaction proceeded smoothly to afford the corresponding allylated product 5a in 77% yield (Table 2, entry 1). To further improve the yield, different palladium(II) salts were screened under established conditions (Table 2, entries 2–6). The best result was obtained using allylpalladium(II) chloride dimer and the desired allylated product 5a was isolated in 94% yield (Table 2, entry 6).
Et2O were employed as catalysts and the allylation of 2a did not occur even at elevated temperature under these conditions (see ESI†). It is reasonable to anticipate that, creation of cation at pseudobenzylic position in the absence of electron rich aromatic system is less favorable. Hence, we employed corresponding 3-OBoc oxindole derivative 3a as the reactant. However, the reaction of trimethylsilane 4a with 3-OBoc oxindole derivative 3a afforded only negligible amount of allylated product 5a under Lewis acid conditions (Table 1, entries 1–6). The allylation reaction between allyltributylstannane 4b and 3-OBoc oxindole derivative 3a afforded the desired product 5a in 38% yield when scandium triflate was employed as a catalyst (Table 1, entry 7). Screening of other Lewis acids under identical conditions did not bring in desired results (entries 8–12). Hence, we turned our attention to palladium catalysts to achieve the allylation at pseudobenzylic position due to their demonstrated potential in C–C bond formation.13 As a preliminary investigation, allylation reaction between allyltributylstannane 4b and 3-OBoc oxindole derivative 3a was carried out in the presence of 5 mol% of palladium(II) chloride and 10 mol% of PPh3 in dichloromethane at ambient temperature. The reaction proceeded smoothly to afford the corresponding allylated product 5a in 77% yield (Table 2, entry 1). To further improve the yield, different palladium(II) salts were screened under established conditions (Table 2, entries 2–6). The best result was obtained using allylpalladium(II) chloride dimer and the desired allylated product 5a was isolated in 94% yield (Table 2, entry 6).
| Entry | Metal salt | Allylating agent 4 | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a The allylation reaction was performed between 3a (1 eq, 0.27 mmol) and 4 (2 eq) with 10 mol% of Lewis acids and 2 equiv. of allylating agents (4a or 4b) in CH2Cl2 as a solvent at rt. b Isolated yield of the product. | ||||
| 1 | Sc(OTf)3 | 4a | 24 | <10 |
| 2 | Cu(OTf)2 | 4a | 24 | — |
| 3 | Yb(OTf)3 | 4a | 24 | Trace |
| 4 | FeCl3 | 4a | 48 | — |
| 5 | SnCl4 | 4a | 48 | — |
| 6 | TiCl4 | 4a | 48 | — |
| 7 | Sc(OTf)3 | 4b | 40 | 38 |
| 8 | Cu(OTf)2 | 4b | 24 | <10 |
| 9 | Yb(OTf)3 | 4b | 48 | 21 |
| 10 | FeCl3 | 4b | 32 | 26 |
| 11 | SnCl4 | 4b | 32 | 30 |
| 12 | TiCl4 | 4b | 32 | 19 |
| Entry | Palladium salt | Time (h) | Yieldb (%) |
|---|---|---|---|
| a The allylation reaction was performed between 3a (1 eq, 0.27 mmol) and 4b (2 eq) with 5 mol% of palladium salt and 10 mol% of PPh3 in CH2Cl2 as a solvent at rt. b Isolated yield of the product. | |||
| 1 | PdCl2 | 15 | 77 |
| 2 | Pd(OAc)2 | 16 | 72 |
| 3 | Pd(PPh3)2Cl2 | 14 | 70 |
| 4 | Pd(TFA)2 | 48 | 60 |
| 5 | Pd2(dba)3 | 48 | 62 |
| 6 | [Pd(η3-C3H5)Cl]2 | 10 | 94 |
Having established the optimal reaction conditions, the influence of nitrogen protecting groups on reactivity was examined. Different N1-substituted 3-OBoc oxindole derivatives 3a–e were subjected to allylation under identical reaction conditions and the results are depicted in Table 3. 3-OBoc oxindoles 3b–e which contain different protecting groups such as benzyl 3b, tert-butyloxy carbonyl 3c, allyl 3d and propargyl 3e rendered the desired allylated products 5b–e with very good yields (84–90%). The presence of different N1-protecting groups did not affect the efficiency of the reaction. Hence, the desired protecting group can be incorporated depending upon the utility of the final product. After examining the effect of protecting groups at N1-position, the impact of having different substitutions at various position on aryl part of the oxindole moiety was studied using N1-methyl substituted 3-OBoc-oxidole 3a.
The effect of halogen substitutions at different positions on aryl ring of oxindole scaffold was investigated initially. It was observed that, sterically demanding 4-Cl and 4-Br substituents are well accommodated under the catalytic conditions to afford the corresponding allylated products (5f and 5g) in good yields.
The allylation reaction of 5-fluoro substituted 3-OBoc oxindole derivative 3h proceeded to yield 5h in 83%, whereas all other 5-halogen substituents rendered the corresponding allylated products 5i–k in very good yields in the range of 90–94%. It is noteworthy that the presence of bromo-5j as well as iodo 5k substituents did not retard the catalytic efficiency of the reaction. Incorporation of electron releasing substitution like 5-methoxy group did not affect the efficiency of the reaction and desired allylated product 5l was isolated with 89% yield. Replacement of 5-methoxy substituent by 5-trifluoromethoxy substitution 5m slightly affected the yield of the reaction (80%). The presence of substitutents at 7-position (7-fluoro, 5,7-dimethyl) did not hamper the catalytic efficiency and resultant allylated products (5n and 5o) were isolated with better yields.
After investigating the influence of various substituents on aryl part of oxindole moiety, scope of differently substituted alkynes was explored. Grafting a methyl substitution either at ortho- or para-position of the aryl ring did not show any negative effect and corresponding allylated products (5p and 5q) were obtained with similar yields (92%). Aryl part of alkyne having bulky group like tert-butyl at para-position underwent smooth allylation to afford the product 5r in 92% yield. Presence of di-fluoro-substitution on aryl part of alkyne moiety did not retard the catalytic efficiency, and the respective o-, p-difluoro substituted product 5s was isolated with 87% yield. To study the effect of presence of hetero atom, 3-(2-pyridyl ethynyl) substituted 3-OBoc-oxindole 3t was employed as a substrate. Under the established catalytic condition, existence of pyridyl group did not pose any adverse effect to the conversion and the product 5t was obtained with an excellent yield of 91%.
The tolerability of alkyl substitution on alkyne moiety was also examined under the optimized conditions. No significant reduction in the yield was observed for substrates 3u and 3v which contain linear aliphatic groups like propyl and –CH2OBn. Trimethylsilyl and cyclopropyl substituted alkynes (3w and 3x) were subjected for similar transformation. They were found to be ideal substrates under established protocol to afford the corresponding allylated products (5w and 5x) in excellent yields (93 and 94%). The presence of double bond in cyclohexenyl substitued alkyne moiety altered neither the pathway nor the efficiency of the reaction. The corresponding product 5y was isolated in good yield (87%). We were delighted to observe that the presence of substitutions like electron-rich and halogen functionalities either at aryl part of oxindole ring or aryl part of alkyne moiety was well tolerated under our established conditions. In addition, replacement of aryl part of alkyne moiety by heteroaryl as well as reactive alkyl substituents did not exert any negative effect on the yield. Scalability of the reaction was evaluated by performing the transformation in 3 gram scale and the expected product 5a was isolated with 87% yield. Thus, we newly developed a palladium(II)-catalyzed C3-allylation of 3-ethynyl-3-OBoc oxindole derivatives 3a–y to construct highly functionalized 3-allyl-3-ethynyl-oxindole scaffolds 5a–y.
To the best of our knowledge, there is no literature precedence of grafting allyl and ethynyl substitutions at C3-position of oxindole. After achieving the synthesis of the same, we wanted to explore application of these intermediates 5 in constructing novel carbocyclic spirooxindoles (Scheme 2). The intermolecular addition reaction between alkyne and alkene is promoted by alkyne–[Co2(CO)6] complex.14 We employed similar strategy to achieve the intramolecular cyclization between alkyne and alkene by generating active acyl cobalt species from alkyne–[Co2(CO)6] complex, which further underwent intramolecular addition with alkene to afford the corresponding spirooxindole cyclohexenone 6a in very good diastereoselectivity (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The observed syn-selectivity for compound 6a is in accordance with XRD data (see ESI†). Under the established conditions, 4-methyl phenyl as well as trimethyl silyl substituted cyclohexenones 6b and 6c were synthesized successfully with diastereomeric ratio of 10
1). The observed syn-selectivity for compound 6a is in accordance with XRD data (see ESI†). Under the established conditions, 4-methyl phenyl as well as trimethyl silyl substituted cyclohexenones 6b and 6c were synthesized successfully with diastereomeric ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 respectively. Substrates which possess propargylic hydrogens e.g.5u afforded a mixture of unidentified inseparable products (cyclised and oxidized products). Hence, the developed catalytic conditions suits well to construct a library of functionalized spiro-4-cyclohexenones 6a–c from aromatic alkynes as well as alkynes which lacks propargylic hydrogens (6c). Pauson–Khand15 reaction condition was applied to synthesize a spiro-bicyclic system from compound 5a. Due to ring strain, the four membered ring formation was not observed 7. Hence, we performed the reaction under the open atmospheric condition to obtain linear carbonylated products (8 and 9).
1 respectively. Substrates which possess propargylic hydrogens e.g.5u afforded a mixture of unidentified inseparable products (cyclised and oxidized products). Hence, the developed catalytic conditions suits well to construct a library of functionalized spiro-4-cyclohexenones 6a–c from aromatic alkynes as well as alkynes which lacks propargylic hydrogens (6c). Pauson–Khand15 reaction condition was applied to synthesize a spiro-bicyclic system from compound 5a. Due to ring strain, the four membered ring formation was not observed 7. Hence, we performed the reaction under the open atmospheric condition to obtain linear carbonylated products (8 and 9).
Next, the intramolecular cyclization reaction of compound 5a under metal free condition was carried out using I2. Treatment of molecular iodine with compound 5a in dichloroethane at 65 °C, furnished the five-membered 2-iodo-4-iodomethyl spiro cyclopentene 10a in good yield (71%) and diasteroselectivity (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). We were pleased to observe that under the optimized conditions, substrates with a range of substitutions including aromatic, trimethylsilyl, aliphatic and hydroxy group underwent smooth cyclization to afford corresponding spiro cyclized products 10b–e in good yields. The stereochemical information was obtained compound 10b from single crystal XRD data and found to be syn with respect to quaternary and tertiary stereocenter (see ESI†). Thus, we have successfully disclosed two unprecedented intramolecular spiro-carbocyclizations with and without metal catalyst. The protocol described here will pave a way to synthesize a library of new spiro-carbocyclic compounds to unravel the hidden truth of cellular processes.
1). We were pleased to observe that under the optimized conditions, substrates with a range of substitutions including aromatic, trimethylsilyl, aliphatic and hydroxy group underwent smooth cyclization to afford corresponding spiro cyclized products 10b–e in good yields. The stereochemical information was obtained compound 10b from single crystal XRD data and found to be syn with respect to quaternary and tertiary stereocenter (see ESI†). Thus, we have successfully disclosed two unprecedented intramolecular spiro-carbocyclizations with and without metal catalyst. The protocol described here will pave a way to synthesize a library of new spiro-carbocyclic compounds to unravel the hidden truth of cellular processes.
Conclusions
To conclude, we established a novel synthetic strategy to access 3-allyl-3-ethynyl substituted oxindole derivatives in very good yields. We employed allylpalladium(II) chloride dimer with PPh3 as a catalyst, to achieve the nucleophilic allylic substitution at pseudobenzylic position with a broad substrate scope. The synthetic utilities of the allylated product were demonstrated with three different applications including the assembly of five and six membered spiro-oxindole carbocyclic scaffolds.Acknowledgements
We acknowledge Indo-French Centre for Promotion of Advanced Research (IFCPAR) (IFC/4803-4), New Delhi for financial support. Kumar thanks CSIR for a research fellowship. We thank Dr Sudha Devi for crystallographic analysis SAIF-IIT Madras and SAIF-IIT Madras for spectral analysis (NMR and IR). We thank department of Biotechnology IIT Madras for LCMS (mass spectrum).Notes and references
- For selected reviews: (a) A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945–2963 CrossRef CAS PubMed; (b) C. Marti and E. M. Carreira, Eur. J. Org. Chem., 2003, 2209–2219, DOI:10.1002/ejoc.200300050; (c) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748–8758 CrossRef CAS PubMed; (d) B. M. Trost and M. K. Brennan, Synthesis, 2009, 3003–3025, DOI:10.1055/s-0029-1216975; (e) A. Millemaggi and R. J. K. Taylor, Eur. J. Org. Chem., 2010, 4527–4547, DOI:10.1002/ejoc.201000643; (f) J. E. M. N. Klein and R. J. K. Taylor, Eur. J. Org. Chem., 2011, 2011, 6821–6841 CrossRef CAS; (g) M. Ishikura, T. Abe, T. Choshi and S. Hibino, Nat. Prod. Rep., 2013, 30, 694–752 RSC.
- Selected references for the 3-hydroxy oxindoles: (a) D. Sano, K. Nagata and T. Itoh, Org. Lett., 2008, 10, 1593–1595 CrossRef CAS PubMed; (b) Y. Yang, F. Moinodeen, W. Chin, T. Ma, Z. Jiang and C.-H. Tan, Org. Lett., 2012, 14, 4762–4765 CrossRef CAS PubMed; (c) D. L. Silverio, S. Torker, T. Pilyugina, E. M. Vieira, M. L. Snapper, F. Haeffner and A. H. Hoveyda, Nature (London, U. K.), 2013, 494, 216–221 CrossRef CAS PubMed; (d) V. Pratap Reddy Gajulapalli, P. Vinayagam and V. Kesavan, Org. Biomol. Chem., 2014, 12, 4186–4191 RSC; Selected references for 3-amino oxindoles; (e) S. P. Marsden, E. L. Watson and S. A. Raw, Org. Lett., 2008, 10, 2905–2908 CrossRef CAS PubMed; (f) E. L. Watson, S. P. Marsden and S. A. Raw, Tetrahedron Lett., 2009, 50, 3318–3320 CrossRef CAS; (g) I. Gorokhovik, L. Neuville and J. Zhu, Org. Lett., 2011, 13, 5536–5539 CrossRef CAS PubMed (h) Y. Li, Y. Shi, Z.-X. Huang, X.-H. Wu, P.-F. Xu, J.-B. Wang and Y. Zhang, Org. Lett., 2011, 13, 1210–1213 CrossRef CAS PubMed; (i) F. Zhu, F. Zhou, Z.-Y. Cao, C. Wang, Y.-X. Zhang, C.-H. Wang and J. Zhou, Synthesis, 2012, 44, 3129–3144 CrossRef CAS.
- Selected references for 3,3 oxindole C–C bond: (a) S. Lee and J. F. Hartwig, J. Org. Chem., 2001, 66, 3402–3415 CrossRef CAS PubMed; (b) J. Xie, P. Xu, H. Li, Q. Xue, H. Jin, Y. Cheng and C. Zhu, Chem. Commun., 2013, 49, 5672–5674 RSC; (c) Y.-H. Jhan, T.-W. Kang and J.-C. Hsieh, Tetrahedron Lett., 2013, 54, 1155–1159 CrossRef CAS; (d) W. Wei, J. Wen, D. Yang, X. Liu, M. Guo, R. Dong and H. Wang, J. Org. Chem., 2014, 79, 4225–4230 CrossRef CAS PubMed.
- (a) B. M. Trost and Y. Zhang, J. Am. Chem. Soc., 2006, 128, 4590–4591 CrossRef CAS PubMed; (b) B. M. Trost, S. Malhotra and W. H. Chan, J. Am. Chem. Soc., 2011, 133, 7328–7331 CrossRef CAS PubMed; (c) B. H. Shupe, E. E. Allen, J. P. MacDonald, S. O. Wilson and A. K. Franz, Org. Lett., 2013, 15, 3218–3221 CrossRef CAS PubMed.
- (a) X. Luan, L. Wu, E. Drinkel, R. Mariz, M. Gatti and R. Dorta, Org. Lett., 2010, 12, 1912–1915 CrossRef CAS PubMed; (b) Y. Zhou, Y. Zhao, X. Dai, J. Liu, L. Li and H. Zhang, Org. Biomol. Chem., 2011, 9, 4091–4097 RSC.
- (a) B. M. Trost and M. U. Frederiksen, Angew. Chem., Int. Ed., 2005, 44, 308–310 CrossRef CAS PubMed; (b) B. M. Trost and Y. Zhang, J. Am. Chem. Soc., 2007, 129, 14548–14549 CrossRef CAS PubMed; (c) B. M. Trost and Y. Zhang, Chem.–Eur. J., 2010, 16, 296–303 CrossRef CAS PubMed; (d) B. M. Trost and Y. Zhang, Chem.–Eur. J., 2011, 17, 2916–2922 CrossRef CAS PubMed; (e) H. Yang, H. Zhou, H. Yin, C. Xia and G. Jiang, Synlett, 2014, 25, 2149–2154 CrossRef CAS.
- L. K. Kinthada, S. Ghosh, S. De, S. Bhunia, D. Dey and A. Bisai, Org. Biomol. Chem., 2013, 11, 6984–6993 CAS.
- (a) M. R. Luzung, J. P. Markham and F. D. Toste, J. Am. Chem. Soc., 2004, 126, 10858–10859 CrossRef CAS PubMed; (b) L. Zhang and S. A. Kozmin, J. Am. Chem. Soc., 2005, 127, 6962–6963 CrossRef CAS PubMed; (c) J. Sun, M. P. Conley, L. Zhang and S. A. Kozmin, J. Am. Chem. Soc., 2006, 128, 9705–9710 CrossRef CAS PubMed; (d) C. M. Grise and L. Barriault, Org. Lett., 2006, 8, 5905–5908 CrossRef CAS PubMed; (e) O. Debleds and J.-M. Campagne, J. Am. Chem. Soc., 2008, 130, 1562–1563 CrossRef CAS PubMed; (f) Y. Horino, T. Yamamoto, K. Ueda, S. Kuroda and F. D. Toste, J. Am. Chem. Soc., 2009, 131, 2809–2811 CrossRef CAS PubMed; (g) A. Pradal, Q. Chen, P. F. Dit Bel, P. Y. Toullec and V. Michelet, Synlett, 2012, 23, 74–79 CAS.
- N. Boechat, W. B. Kover, V. Bongertz, M. M. Bastos, N. C. Romeiro, M. L. G. Azevedo and W. Wollinger, Med. Chem., 2007, 3, 533–542 CrossRef CAS.
- (a) F. Yang, G. Zhao and Y. Ding, Tetrahedron Lett., 2001, 42, 2839–2841 CrossRef CAS; (b) M. R. Luzung and F. D. Toste, J. Am. Chem. Soc., 2003, 125, 15760–15761 CrossRef CAS PubMed; (c) T. Schwier, M. Rubin and V. Gevorgyan, Org. Lett., 2004, 6, 1999–2001 CrossRef CAS PubMed; (d) M. Georgy, V. Boucard and J.-M. Campagne, J. Am. Chem. Soc., 2005, 127, 14180–14181 CrossRef CAS PubMed; (e) Z.-P. Zhan, J.-L. Yu, H.-J. Liu, Y.-Y. Cui, R.-F. Yang, W.-Z. Yang and J.-P. Li, J. Org. Chem., 2006, 71, 8298–8301 CrossRef CAS PubMed; (f) R. Sanz, A. Martinez, J. M. Alvarez-Gutierrez and F. Rodriguez, Eur. J. Org. Chem., 2006, 1383–1386, DOI:10.1002/ejoc.200500960; (g) G. Kabalka, S. Borella and M.-L. Yao, Synthesis, 2008, 325–329, DOI:10.1055/s-2007-990816; (h) J. S. Yadav, B. V. S. Reddy, T. S. Rao and K. V. R. Rao, Tetrahedron Lett., 2008, 49, 614–618 CrossRef CAS; (i) M. Georgy, V. Boucard, O. Debleds, C. Dal Zotto and J.-M. Campagne, Tetrahedron, 2009, 65, 1758–1766 CrossRef CAS; (j) M. Lin, L. Hao, X.-T. Liu, Q.-Z. Chen, F. Wu, P. Yan, S.-X. Xu, X.-L. Chen, J.-J. Wen and Z.-P. Zhan, Synlett, 2011, 665–670, DOI:10.1055/s-0030-1259557; (k) K. Murugan and C. Chen, Tetrahedron Lett., 2011, 52, 5827–5830 CrossRef CAS; (l) D. Das, S. Pratihar, U. K. Roy, D. Mal and S. Roy, Org. Biomol. Chem., 2012, 10, 4537–4542 RSC.
- (a) Z.-P. Zhan, J.-L. Yu, H.-J. Liu, Y.-Y. Cui, R.-F. Yang, W.-Z. Yang and J.-P. Li, J. Org. Chem., 2006, 71, 8298–8301 CrossRef CAS PubMed; (b) P. N. Chatterjee and S. Roy, J. Org. Chem., 2010, 75, 4413–4423 CrossRef CAS PubMed; (c) V. J. Meyer and M. Niggemann, Eur. J. Org. Chem., 2011, 2011, 3671–3674 CrossRef CAS; (d) G. G. K. S. Narayana Kumar and K. K. Laali, Org. Biomol. Chem., 2012, 10, 7347–7355 RSC.
- (a) X.-P. Fu, L. Liu, D. Wang, Y.-J. Chen and C.-J. Li, Green Chem., 2011, 13, 549–553 RSC; (b) G. Chen, Y. Wang, S. Gao, H.-P. He, S.-L. Li, J.-X. Zhang, J. Ding and X.-J. Hao, J. Heterocycl. Chem., 2009, 46, 217–220 CrossRef CAS.
- (a) M. Yoshida, M. Fujita and M. Ihara, Org. Lett., 2003, 5, 3325–3327 CrossRef CAS PubMed; (b) X.-H. Duan, L.-N. Guo, H.-P. Bi, X.-Y. Liu and Y.-M. Liang, Org. Lett., 2006, 8, 5777–5780 CrossRef CAS PubMed; (c) M. Yoshida, M. Higuchi and K. Shishido, Org. Lett., 2009, 11, 4752–4755 CrossRef CAS PubMed; (d) M. Yoshida, T. Nakagawa, K. Kinoshita and K. Shishido, J. Org. Chem., 2013, 78, 1687–1692 CrossRef CAS PubMed; (e) G. Frensch, N. Hussain, F. A. Marques and P. J. Walsh, Adv. Synth. Catal., 2014, 356, 2517–2524 CrossRef CAS PubMed; (f) S. Montel, L. Raffier, Y. He and P. J. Walsh, Org. Lett., 2014, 16, 1446–1449 CrossRef CAS PubMed; (g) S. Montel, T. Jia and P. J. Walsh, Org. Lett., 2014, 16, 130–133 CrossRef CAS PubMed.
- I. Ooi, T. Sakurai, J. Takaya and N. Iwasawa, Chem.–Eur. J., 2012, 18, 14618–14621 CrossRef CAS PubMed.
- (a) S. E. Gibson and A. Stevenazzi, Angew. Chem., Int. Ed., 2003, 42, 1800–1810 CrossRef CAS PubMed; (b) L.-L. Shi, H.-J. Shen, L.-C. Fang, J. Huang, C.-C. Li and Z. Yang, Chem. Commun., 2013, 49, 8806–8808 RSC; (c) J. Huang, L. Fang, R. Long, L.-L. Shi, H.-J. Shen, C.-C. Li and Z. Yang, Org. Lett., 2013, 15, 4018–4021 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1021476, 1022902 and 1035651. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra07955a |
| This journal is © The Royal Society of Chemistry 2015 |