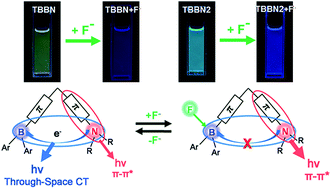
A new class of Λ-shaped triarylboranes 2-(4-(N,N-dimethylamino)-8-dimesitylboryl-6H,12H-5,11-methanodibenzo[b,f][1,5] diazocine (TBBN) and 2-(4-(N,N-diphenylamino)-8-dimesitylboryl-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine (TBBN2), incorporating different electron-donating amino groups and an electron-accepting dimesitylboryl group through a rigid Λ-shaped Tröger's base linker were designed and synthesized. The compounds display twisted structures and effective intramolecular change-transfer transitions. The twisted nonplanar arrangement of the chromophores on the one hand suppresses the fluorescence quenching in the aggregated states, and on the other hand produces a through-space donor–acceptor charge transfer. As a result, dual fluorescent pathways, namely through-space charge transfer from the amino group to the dimesitylboryl group, and the π*–π transitions located on the amino groups, are observed to coexist in each molecule. The dual emissions can be selectively switched on or off by addition of fluoride or cyanide ions. Thus the dyes can be used as “switch-on” probes. The complexation of TBBN and TBBN2 with fluoride or cyanide ions induces dramatic blue shifts (about 72–140 nm) and color changes in the fluorescence, making them potential visually colorimetric and ratiometric sensors for fluoride and cyanide ions.