Non-corroding α-alumina@TiO2 core–shell nanoplates appearing metallic gold in colour
Myung Won Suh†
a,
Su Jin Lee†b,
Myoung Sang Youb,
Seung Bin Park*a and
Sang Hyuk Im*b
aDepartment of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology (KAIST), 291 Daehak-ro, Yuseong-gu, Daejeon 305-701, Republic of Korea. E-mail: sbpark7@kaist.ac.kr
bFunctional Crystallization Center (FCC), Department of Chemical Engineering, Kyung Hee University, 1732 Deogyeong-daero, Giheung-gu, Yongin-si, Gyeonggi-do 446-701, Republic of Korea. E-mail: imromy@khu.ac.kr
First published on 18th June 2015
Abstract
Non-corroding α-alumina@TiO2 core–shell nanoplates exhibiting a lustrous metallic gold colour were synthesized using sol–gel solution chemistry with controlled reaction conditions for the formation of anatase and rutile TiO2 nanocrystals. Due to the small mismatch of lattice parameters between rutile TiO2 and two-dimensional α-alumina nanoplates, the rutile TiO2 could be conformably grown on the surface of the α-alumina nanoplates, however, the lustring effect was deteriorated by back scattering due to the formation of rough needle-shaped rutile TiO2. The surface roughness of the TiO2 nano-shell was controlled by controlling the pH of the reactant solution because rutile phase TiO2 nanocrystals tend to be formed in more acidic conditions, whereas anatase phase TiO2 is likely to be promoted in less acidic conditions, of which anatase small nanocrystals smooth the surface of the TiO2 nano-shell during the crystal growth. The successful formation of a smooth TiO2 nano-shell via controlled crystal growth was associated with the fact that the intermediate complex of [Ti(OH)mCln]2− (m + n = 6) tends to cause corner sharing in more acidic conditions and edge sharing in less acidic conditions. Apparently the α-alumina@TiO2 core–shell nanoplates prepared in more and less acidic conditions showed a metallic gold colour, however, the core–shell nanoplates synthesized in less acidic conditions exhibited a better lustring effect than those from the more acidic conditions due to the formation of a smooth TiO2 shell.
Introduction
Two-dimensional (2D) nanoplates such as graphene, metals, semi-conductors, and metal oxides have been of great interest because of their potential for application in flexible transparent electrodes,1 barrier films,2 thin film transistors,3 stretchable electrodes,4 and colour pigments.5 Among them, metal oxide 2D nanoplates including glass,6 mica,7 and alumina nanoflakes8 have been extensively utilized in the pigment industry because they can show a lustring colour and the colour can be controlled by the coating thickness and refractive index of shell material. Hence, the lustring colour is originated from the constructive and deconstructive interference of light at the air/shell/2D metal oxide nanoplate interface, thereby exhibiting angle dependent structure colouring like one-dimensional photonic crystals. For instance, if we assume that the incident light with θ angle from the normal direction is reflected at the shell (s)/2D metal oxide nanoplate (mo) interface, the constructive interference occurs when it satisfies following condition:9
2nsd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = mλ θ = mλ
| (1) |
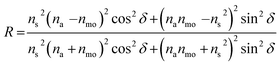 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ, respectively. Therefore, the colour of the reflected light is dependent on the thickness (d), refractive index (ns), and incident angle (θ) of the shell. Moreover, the intensity of the reflected light is closely related to the refractive index of each layer and the refractive index difference between each layer. In particular, here the na and nm are fixed for air (na = 1.0) and α-alumina (nmo = 1.7) so that the ns is important for maximizing the lustring effect. Namely, higher refractive index materials such as crystalline TiO2 are more desirable for shell materials to show a better lustring effect.
θ/λ, respectively. Therefore, the colour of the reflected light is dependent on the thickness (d), refractive index (ns), and incident angle (θ) of the shell. Moreover, the intensity of the reflected light is closely related to the refractive index of each layer and the refractive index difference between each layer. In particular, here the na and nm are fixed for air (na = 1.0) and α-alumina (nmo = 1.7) so that the ns is important for maximizing the lustring effect. Namely, higher refractive index materials such as crystalline TiO2 are more desirable for shell materials to show a better lustring effect.
Initially, pearlescent 2D pigments were obtained from the fish scales of hairtails or herrings because the synthetic technology for artificial 2D pigments was not developed. Although crystalline 2D guanine or hypoxanthine natural pigments can be collected from fish scales, the yield was very low and insufficient to cope with the demands for pearlescent pigments. Therefore, artificial 2D pearlescent pigments such as basic lead carbonate (Pb(OH)2·2PbCO3), bismuth oxychloride (BiOCl), glass, and mica have been synthesized.5 Among them, the 2D mica pigments have been widely used until now because natural mica could be easily collected from mines. However, natural mica is often revealed to be of non-uniform colour or yellowish in colour because the composition of the impurities in natural mica differs depending on the location of the mine. Mica is composed of complex hydrous potassium-aluminium silicate and consequently has various names such as biotite, lepidolite, muscovite, phlogopite, and vermiculite according to its chemical composition.7a Therefore, mica exhibits different colours according to the variation in its chemical composition. In addition, mica requires additives to coat TiO2 on its surface and the control of the surface roughness of the TiO2 shell is difficult, as well.7c,d Accordingly, 2D α-alumina nanoplates will be better as a mother substrate than mica because α-alumina has a pure phase and small lattice mismatch with rutile TiO2. Here, we try to coat a TiO2 nano-shell on the surface of α-alumina nanoplates in order to synthesize non-corroding pigments with a metallic gold colour because TiO2 is a very stable metal oxide which is not corroded by most acidic or basic chemicals.
Experimental
Materials
2D α-alumina nanoplates were purchased from TCERA. TiCl4 (titanium tetrachloride), HCl, and NaOH were purchased from Fluka, Samchun, and Samchun, respectively. We used the chemicals as received without further purification.Characterization
For the analysis using scanning electron microscopy (SEM: Supra 55, Carl Zeiss) images of the samples, we dropped a 2D α-alumina nanoplate, 2D α-alumina@TiO2 core–shell nanoplate or TiO2 nanoparticle dispersion solution on a Si wafer and dried it. The dried samples were then coated with Pt by sputtering. For the analysis using transmission electron microscopy (TEM: Jem-2100F, Jeol) images, we dropped the TiO2 nano-sol solution on a carbon coated Cu grid. For the analysis using X-ray diffraction (XRD: D8 advance, Bruker) patterns, we collected the dried powder of the 2D α-alumina nanoplates or 2D α-alumina@TiO2 core–shell nanoplates or TiO2 nanoparticles and placed it on a Si low background sample holder. The samples were scanned from 20° to 80° at a scan rate of 6° min−1 under the irradiation of Cu K-α (λ = 0.15406 nm).Results and discussion
The scanning electron microscopy (SEM) image of the 2D α-alumina nanoplates in Fig. 1(a) indicates that the lateral size and thickness of the nanoplates is 10–30 μm and ∼500 nm, respectively. Fig. 1(b) is a magnified SEM image of the white rectangle area in Fig. 1(a), which indicates that the surface of the nanoplate is uniform. This indicates that the 2D nanoplates are adaptable for use as substrates for interference pearlescent pigments. Fig. 1(c) is a photograph of the 2D α-alumina nanoplates which were prepared by dropping a 2D α-alumina nanoplate/water dispersion solution on a glass plate and subsequent drying. The pristine 2D α-alumina nanoplates exhibited a metallic silver colour. Fig. 1(d) shows X-ray diffraction (XRD) patterns of the 2D nanoplates, indicating that the nanoplates have a crystalline α-alumina phase.Table 1 summarizes the material properties of crystalline α-Al2O3, rutile TiO2, and anatase TiO2 indicating that the lattice parameter of rutile TiO2 is slightly mismatched with the lattice parameter of α-Al2O3, whereas the anatase TiO2 has great lattice mismatch with the α-Al2O3. In addition, the refractive index of rutile TiO2 is higher than for anatase TiO2 so the lustring effect is more enhanced by rutile TiO2. Therefore, here, we try to coat a rutile TiO2 nano-shell on the surface of 2D α-alumina nanoplates in order to create a pearlescent metallic gold colour.
| Properties | α-Al2O3 | Rutile | Anatase |
|---|---|---|---|
| Crystal system | Hexagonal | Tetragonal | Tetragonal |
| a | 4.75 Å | 4.59 Å | 3.78 Å |
| b | — | 4.59 Å | 3.78 Å |
| c | 12.99 Å | 2.96 Å | 9.51 Å |
| Density | 3.95–4.03 | 4.3 | 3.8 |
| Refractive index | 1.7 | 2.7 | 2.5 |
To find the formation conditions of rutile TiO2 in acidic conditions, we conducted experiments adding 0.5 mL of 3 M TiCl4 aqueous solution and a HCl solution (0, 0.1, 0.2, 0.5, and 1 mL) into 20 mL of deionized water in a vial and subsequently reacting them at 60 °C for 1 h under magnetic stirring. The XRD patterns of the produced TiO2 particles in Fig. 2(a) show that the pure rutile TiO2 phase is formed in more acidic conditions (HCl = 0.5 and 1 mL under the current experimental conditions) and the anatase TiO2 phase is dominantly formed in less acidic conditions (HCl = 0.2, 0.1 and 0 mL under the current experimental conditions). This tendency is well known because 3 M TiCl4 aqueous solution forms stable TiOCl2 and the diluted TiOCl2 begins to form [Ti(OH)mCln]2− (m + n = 6), which tends to create corner sharing by condensation reaction in more acidic conditions due to more existence of Cl−, whereas it creates edge sharing in less acidic conditions.10 Fig. 2(b) is the TEM (transmission electron microscopy) image of the produced TiO2 particles from less acidic conditions (HCl = 0 mL) indicating that small anatase TiO2 nanocrystals with ∼5 nm in size were synthesized. Fig. 2(c) is the SEM image of the produced TiO2 particles from more acidic conditions (HCl = 1 mL) indicating the formation of relatively larger needle-shaped rutile TiO2 primary nanoparticles with ∼10 nm in size. These results imply that the rutile TiO2 nano-shell can be directly coated on the 2D α-alumina nanoplates due to a small mismatch of the lattice parameter in more acidic conditions, but a rough surface will be formed. Meanwhile a smooth surface can be formed in less acidic conditions due to the formation of small anatase TiO2 nanocrystals. Therefore, the coating process needs to be started in more acidic conditions at early reaction stages and finished in less acidic conditions at late stages in order to coat a smooth TiO2 nano-shell with small surface roughness on the 2D α-alumina nanoplates. To confirm the crystal size of the synthesized TiO2 nanoparticles, we calculated the representative crystal size of the anatase TiO2 (HCl – 0 mL) and rutile TiO2 (HCl – 1 mL) samples from the Scherrer equation:11
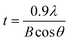 | (3) |
To check if the rutile TiO2 can be directly coated on the 2D α-alumina nanoplates, we coated TiO2 nano-shells in more acidic conditions by adding 0.05 g of the 2D α-alumina nanoplates, 1 mL of HCl solution, and 0.5 mL of 3 M TiCl4 aqueous solution into 20 mL of deionized water in a vial and subsequently heating at 98 °C for 2 h under magnetic stirring. To check the effects of pre-treatment of the 2D α-alumina nanoplates in acidic solution, we conducted again the above experiment by stirring a solution mixture of 0.05 g of the 2D α-alumina nanoplates, 1 mL of HCl solution, and 20 mL of deionized water for 2 h and then reacting it at 98 °C for 2 h after adding 0.5 mL of 3 M TiCl4 aqueous solution into the solution mixture. The SEM images of the 2D α-alumina nanoplates coated by TiO2 with and without HCl pre-treatment are shown in Fig. 3. They clearly indicate that the HCl pre-treatment is effective for forming a full covering of TiO2 nano-shell on the surface of the 2D α-alumina nanoplates because all the 2D α-alumina nanoplates were coated by TiO2 nano-shells in the HCl pre-treated sample (Fig. 3(a)), whereas some 2D α-alumina nanoplates were not fully covered by TiO2 nano-shells in the sample without HCl pre-treatment (Fig. 3(b) and see sample area marked by arrows). The inset in Fig. 3(b) is a magnified SEM image of the uncovered part indicating the existence of incomplete TiO2 coating.
To provide a metallic gold structure colouring effect to the 2D α-alumina nanoplates, we formed a TiO2 rutile and anatase mixture coating layer on the surface of the 2D α-alumina to see if a smoother TiO2 shell can be formed or not. For this, we pre-treated the 2D α-alumina nanoplates by stirring a 0.05 g α-alumina nanoplates/20 mL deionized water/1 mL HCl solution mixture for 2 h and adding 0.5 mL of 3 M TiCl4 solution into the solution at 98 °C. 0.1 mL of 0.8 M NaOH aqueous solution was added into the reactant solution 25 times consecutively and the mixture was reacted for 2 h. Then 0.1 mL of 0.8 M NaOH aqueous solution was again added into the reactant solution 25 times consecutively and the mixture was reacted for 2 h. The SEM image of the synthesized 2D α-alumina@TiO2 core–shell nanoplates is shown in Fig. 4(a) indicating that a smoother TiO2 shell was formed using the modified reaction than the TiO2 shell formed in Fig. 3(a) because the rough needle-shaped rutile TiO2 shell is likely to be formed in more acidic conditions, whereas the smooth particulate anatase TiO2 shell tends to be co-formed with rutile TiO2 during pH adjustment by the addition of NaOH. Therefore, rutile TiO2 might be preferentially grown on the surface of the 2D α-alumina nanoplates and the mixture of rutile and anatase TiO2 might be co-grown on the TiO2 layer, thereby reducing the surface roughness of the TiO2 shell. The magnified SEM image in Fig. 4(b) shows that the surface morphology is composed of needle-shaped nanoparticles and irregular smaller nanoparticles. The photograph of the dried 2D α-alumina@TiO2 core–shell nanoplates is shown in Fig. 4(c) confirming the successful formation of a TiO2 nano-shell on the 2D α-alumina nanoplates due to the appearance of gold structure colouring, whereas the pristine 2D α-alumina nanoplates exhibit a silver colour. Fig. 4(d) is the corresponding XRD pattern of the 2D α-alumina@TiO2 core–shell nanoplates indicating that the TiO2 shell is composed of a mixed phase of rutile and anatase TiO2 nanocrystals as we intended. The calculated crystal size of the anatase and rutile TiO2 in the shell from the Scherrer equation was ∼8 nm and ∼16 nm, respectively. The crystalline phase composition of the TiO2 shell can be calculated from the integrated intensity of the (101) peak for anatase and the (110) peak for rutile using the following equation:12
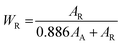 | (4) |
Although the surface roughness of the TiO2 shell can be reduced using the reaction design for co-formation of a rutile and anatase TiO2 shell, the surface roughness needs to be more smooth in order to enhance the lustring effect. Hence, we increased the amount of added NaOH in the reaction and additionally added the Ti precursor under less acidic conditions to promote the formation of anatase TiO2 nanocrystals. For this, we stirred a 0.05 g α-alumina nanoplates/20 mL deionized water/1 mL HCl solution mixture for 2 h for the pre-treatment process and 0.25 mL of 3 M TiCl4 aqueous solution was added into the reactant solution at 98 °C. 0.1 mL of 0.8 M NaOH aqueous solution was added into the reactant solution 37 times consecutively and the mixture was reacted for 1 h. Then 0.25 mL of 3 M TiCl4 aqueous solution was added and subsequently 0.1 mL of 0.8 M NaOH aqueous solution was again added into the reactant solution 37 times consecutively and the mixture was reacted for 1 h. Finally, we added again 0.25 mL of 3 M TiCl4 aqueous solution into the reaction solution and added 37 times by consecutive addition 0.1 mL of 0.8 M NaOH aqueous solution. The reaction was finished after additional reaction for 2 h. The SEM image of the produced 2D α-alumina@TiO2 core–shell nanoplates is shown in Fig. 5(a) and its magnified image is shown in Fig. 5(b) indicating that a smoother TiO2 shell was formed using the modified reaction process. The photograph of the dried 2D α-alumina@TiO2 core–shell nanoplates in Fig. 5(c) exhibited a more intense gold colour due to the reduced back scattering. Fig. 5(d) confirms that the composition of the TiO2 shell is composed of anatase and rutile phases. As we expected, the calculated WR is ∼0.22 indicating that crystalline anatase TiO2 is formed more than in the above experimental result (see Fig. 4). Accordingly, the smoother TiO2 nano-shell was formed by the greater formation of anatase TiO2 nanocrystals. As expected, the synthesized 2D α-alumina@TiO2 core–shell nanoplates were not corroded by acidic (except HF solution) or basic chemicals because the TiO2 shell is a very stable metal oxide.
Conclusions
We could successfully synthesize non-corroding 2D α-alumina@TiO2 core–shell nanoplates showing a metallic gold colour via sol–gel solution chemistry with controlled reaction conditions for the formation of anatase and rutile TiO2 nanocrystals. When we used a 0.5 mL of 3 M TiCl4 aqueous solution/0, 0.1, 0.2, 0.5, or 1 mL of HCl solution/20 mL deionized water solution mixture, the rutile phase of TiO2 was formed in more acidic conditions (HCl = 0.5 and 1 mL) and the anatase phase of TiO2 was formed in less acidic conditions (HCl = 0.2, 0.1 and 0 mL) because the [Ti(OH)mCln]2− (m + n = 6) intermediate complex tends to form corner sharing in more acidic conditions due to the greater existence of Cl− and creates edge sharing in less acidic conditions. Due to the small lattice mismatch between rutile TiO2 and the α-alumina nanoplates, rutile TiO2 could be conformably grown on the surface of the α-alumina nanoplates. However, the rutile TiO2 shell exhibited a rough surface due to the formation of rough needle-shaped crystals and consequently reduced the lustring effect due to a back scattering effect. The surface roughness of the TiO2 nano-shell could be further reduced using a controlled reaction for co-formation of rutile and anatase TiO2 nanocrystals during the TiO2 coating. The successful coating of a smooth TiO2 nano-shell on the 2D α-alumina nanoplates was associated with the fact that the rutile TiO2 nanocrystals are preferentially coated on the 2D α-alumina nanoplates and the smaller irregular sphere-shaped anatase TiO2 nanoparticles are co-formed on the surface of the α-alumina while smoothing the surface roughness of the TiO2 nano-shell. We believe that this controlled reaction method to form a smooth inorganic TiO2 nano-shell is helpful for the synthesis of opalescent colour pigments, angle dependent structure colour pigments, safety pigments, and cosmetic pearls.Acknowledgements
This study was supported by the Basic Science Research Program (no. 2014R1A5A1009799) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning.References
- (a) K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J.-H. Ahn, P. Kim, J.-Y. Choi and B. H. Hong, Nature, 2009, 457, 706 CrossRef CAS PubMed; (b) S. Bae, H. Kim, Y. Lee, X. Xu, J.-S. Park, Y. Zheng, J. Balakrishnan, T. Lei, H. R. kim, Y. I. Song, Y.-J. Kim, K. S. Kim, B. Özyilmaz, J.-H. Ahn, B. H. Hong and S. Ijima, Nat. Nanotechnol., 2010, 5, 574 CrossRef CAS PubMed; (c) J. Wu, H. A. Becerril, Z. Bao, Z. Liu, Y. Chen and P. Peumans, Appl. Phys. Lett., 2008, 92, 263302 CrossRef PubMed.
- (a) H. Kim, Y. Miura and C. W. Macosko, Chem. Mater., 2010, 22, 3441 CrossRef CAS; (b) Y. Su, V. G. Kravets, S. L. Wong, J. Waters, A. K. Geim and R. R. Nair, Nat. Commun., 2014, 5, 4843 CrossRef CAS PubMed.
- (a) B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147 CrossRef CAS PubMed; (b) S.-K. Lee, H. Y. Jang, S. Jang, E. Choi, B. H. Hong, J. Lee, S. Park and J.-H. Ahn, Nano Lett., 2012, 12, 3472 CrossRef CAS PubMed; (c) C. Yan, J. H. Cho and J.-H. Ahn, Nanoscale, 2012, 4, 4870 RSC.
- (a) G. D. Moon, G.-H. Lim, J. H. Song, M. Shin, T. Yu, B. Lim and U. Jeong, Adv. Mater., 2013, 25, 2707 CrossRef CAS PubMed; (b) Y. G. Seol, T. Q. Trung, O.-J. Yoon, I.-Y. Sohn and N.-E. Lee, J. Mater. Chem., 2012, 22, 23759 RSC.
- M. Debeljak, A. Hladnik, L. Cerne and D. Gregor-Svetec, Color Res. Appl., 2012, 38, 168 CrossRef PubMed.
- G. Pfaff, Inorg. Mater., 2003, 39, 123 CrossRef CAS.
- (a) V. Štengl, J. Šubrt, S. Bakardjieva, A. Kalendova and P. Kalenda, Dyes Pigm., 2003, 58, 239 CrossRef; (b) P. M. T. Cavalcante, M. Dondi, G. Guarini, F. M. Barros and A. B. Luz, Dyes Pigm., 2007, 74, 1 CrossRef CAS PubMed; (c) M. Ren, H. Yin, A. Wang, T. Jiang and Y. Wada, Mater. Chem. Phys., 2007, 103, 230 CrossRef CAS PubMed; (d) Q. Gao, X. Wu, Y. Fan and X. Zhou, Dyes Pigm., 2012, 95, 534 CrossRef CAS PubMed.
- (a) S. Teaney, G. Pfaff and K. Nitta, Eur. Coat. J., 1999, 4, 90 Search PubMed; (b) S. Sharrock and N. Schuel, Eur. Coat. J., 2000, 1/2, 20 Search PubMed.
- E. Hecht, Optics, Pearson Education, Inc., Addison Wesley, 4th edn, 2001, ch. 4 and 9 Search PubMed.
- H. Cheng, J. Ma, Z. Zhao and L. Qi, Chem. Mater., 1995, 7, 663 CrossRef CAS.
- B. D. Cullity and S. R. Stock, Elements of X-ray diffraction, Pearson, 2001 Search PubMed.
- (a) J. Yu, Y. Su and B. Cheng, Adv. Funct. Mater., 2007, 17, 1984 CrossRef CAS PubMed; (b) T. H. Han, H.-S. Moon, J. O. Hwang, S. I. Seok, S. H. Im and S. O. Kim, Nanotechnology, 2010, 21, 185601 CrossRef PubMed.
Footnote |
| † M. W. Suh and S. J. Lee contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |





