Magnetic nano-sized cadmium ferrite as an efficient catalyst for the degradation of Congo red in the presence of microwave irradiation
Wen Shi,
Xueyan Liu,
Tingting Zhang,
Qiong Wang and
Lei Zhang*
College of Chemistry, Liaoning University, 66 Chongshan Middle Road, Shenyang 110036, People's Republic of China. E-mail: zhanglei63@126.com; Fax: +86 24 62202380; Tel: +86 24 62207809
First published on 2nd June 2015
Abstract
A highly active nano-sized CdFe2O4 catalyst was prepared by a hydrothermal process and characterized by X-ray diffraction (XRD), scanning electronic microscopy (SEM), BET specific surface area method, and vibrating sample magnetometer (VSM) at room temperature. Application of the microwave-induced catalytic degradation method in the abatement of Congo red (CR) using the magnetic catalyst was studied. The degradation ratio of CR with CdFe2O4 reached 94.4% with 10 min microwave irradiation (MW), proving CdFe2O4 to be an excellent microwave catalyst. The intermediate products from CR degradation were investigated by UV-Vis, HPLC and ion chromatography. The reaction kinetics, effects of different ion species (SO42−, NO3−, HCO3−, and CH3COO−), pH of the solution, dye initial concentration, dosage of catalyst, as well as degradation mechanism were comprehensively studied. Radical trapping studies and the fluorescence technique revealed the holes (h+) and hydroxyl radicals (˙OH) were involved as the main active species in the reaction. The band structure of CdFe2O4 was analyzed by UV-Vis diffuse reflectance spectroscopy and Mott–Schottky measurements. The mechanism of the degradation was discussed in detail. This work can provide an effective technology for dye wastewater treatment.
1. Introduction
Synthetic dyestuffs are used extensively in textile, paper-making, and printing industries and dyehouses. The effluents of these industries are highly colored and the disposal of these wastes into receiving waters can cause damage to the environment. The dyes generally have complex aromatic structures and thus most of them are highly resistant to breakdown by chemical, physical, and biological treatments.1,2 Microwave techniques have been applied in various fields, such as organic synthesis,3,4 analysis,5 and military and environmental engineering.6,7 Researchers have focused on the application of MW irradiation in environmental wastewater treatment,8–10 particularly in the degradation of dyes with complex aromatic structures, which are toxic and nondegradable.11,12 The microwave-assisted degradation technique for pollutants can potentially reduce reaction time.Over the past decade, MW absorbing materials, known as dielectrics, have been the focus of much attention, particularly for their significant roles in wastewater treatment. Materials, such as activated carbon,13,14 transition metal oxide,15,16 CNTs17,18 and polymers,19 are commonly used in microwave-assisted degradation of organic pollutants. Among MW absorbents, ferrites are the most promising.20 Ferrite, which derived from iron oxides, has attracted great interest for its strong absorption ability of microwave. Some ferrites as MeFe2O4 (Me = Zn, Mn, Co, Ni, Cu, Mg, etc.), appeared to be especially efficient for the degradation and removal of organic pollutants.21–26 However, the performance of nanoscale ferrites in MW-induced degradation process is still needed further research.
Now, we were interested in exploring another member of ferrites, magnetic CdFe2O4. CdFe2O4 has normal spinel structure, excellent gas-sensing properties,27 better charge transport characteristics and small bandgap (1.97 eV).28 Within literature, most of the studies discussed its applications as gas-sensing materials, but much less attention is given for dye degradation systems. To the best of our knowledge, there is no report on the systematic investigation on dye degradation using nano-sized magnetic CdFe2O4 as MW absorbing material.
In the present work, CdFe2O4 was prepared by a one-step hydrothermal method. The catalytic activities of CdFe2O4 were investigated by degradation of CR solution under MW. The focus was concentrated on below aspects: (1) exploring potential possibility about MW-induced CdFe2O4 catalytic degradation of dye; (2) identifying intermediates via HPLC and ionic chromatogram techniques and proposing MW-induced degradation mechanism based on the exploration of generating chemically-active species.
2. Experimental section
2.1. Chemicals
The target dye CR was purchased from Sinopharm Chemical Reagent Co. Ltd., and used without further purification. All chemicals used in this experiment were analytical grade. Deionized water was used throughout the experiments.2.2. Synthesis and characterization of CdFe2O4
CdFe2O4 nanoparticles were synthesized using a hydrothermal process. Cd(NO3)2·4H2O (1.2339 g, 0.004 mol) and Fe(NO3)3·9H2O (3.2320 g, 0.008 mol) were dissolved in 80 mL of ethanol under continuous stirring to get a homogeneous suspension. The solution was then transferred into a 100 mL Teflon lined stainless-steel autoclave, which was sealed and maintained at 180 °C for 10 h. After allowing the reactor to cool down to room temperature, the precipitate was separated from solution by centrifugation, washed with deionized water and dried at 60 °C for 12 h.The morphologies of the catalyst were characterized using scanning electron microscopy (HITACHI SU8000). Measurement of BET surface area was performed using N2 adsorption–desorption isotherms on a Micromeritics (Norcross, GA). The X-ray diffraction (XRD) patterns of CdFe2O4 powders were recorded on Siemens D5000 Diffractometer (Germany). Vibrating sample magnetometer (VSM, Lakeshore 7407) was used to measure the magnetic prosperities of CdFe2O4. A Malvern Zetasizer Nano-ZS particle analyzer (Malvern, U.K.) was used to determine the ζ potential of CdFe2O4. UV-Vis diffuse reflectance spectrum (DRS) was acquired by a UV2550 (Shimadzu Scientific Instruments Inc. Japan) and BaSO4 was used as the reflectance standard. A conventional three electrode cells using a CHI660D electrochemical workstation (Shanghai Chenhua, China) was used to determine the flat-band potential (Vfb) of the sample. The working electrode was prepared by dip-coating method as following: 10 mg sample was suspended in 10 mL deionized water, which was then dip-coated onto a 1 cm × 1 cm fluorine–tin oxide (FTO) conducting glass electrode, while a platinum wire as counter electrode, and a standard Ag/AgCl in saturated KCl as reference electrode. The Mott–Schottky measurements were carried out from −1.0V to 1.0V.
2.3. Degradation tests and analytical methods
The experiments were carried out in a temperature-controllable microwave oven (XH100B, Beijing XiangHu Ltd. China) equipped with a reflux condenser. At the beginning, 50 mL of CR (20 mg L−1) aqueous solution and 0.05 g of CdFe2O4 were added into a 250 mL round bottom flask with 3 necks. Then set up the parameters of the MW oven: power (700 W), temperature (100 °C). CR solution samples during the degradation were taken out at predetermined time intervals by magnetic separation. The degradation of CR under MW irradiation without any catalysts and the CR adsorption on CdFe2O4 without MW irradiation were also carried out for comparison.Cary 5000 UV-Vis-NIR (Varian, USA) was utilized to record the spectra of aqueous CR solution after treatment. The reaction intermediates were detected by HPLC (Agilent 1100, USA) equipped with diode array detector and a column oven. A 150 mm × 4.6 mm reverse-phase C-18 column was used for separation. The injection volume was 20 μL, flow rate was 1.0 mL min−1, UV detector wavelength was 497 nm and column oven temperature maintained at 30 °C. The compounds were eluted with methanol–water (38/62 (v/v)).
For further validating degradation of CR in aqueous solution, the ionic chromatogram was used. The other conditions were as follows: AS9-HC column (250 mm × 4 mm i.d.), 9.0 mmol L−1 Na2CO3 eluent, 1.0 mL min−1 flow rate and conductivity detector.
2.4. Analysis of hydroxyl radicals
The formation of hydroxyl radicals on the surface is measured by the fluorescence method, which uses terephthalic acid as a probe molecule.21,29 Terephthalic acid readily reacted with hydroxyl radicals to produce highly fluorescent product, 2-hydroxyterephthalic acid. The intensity of the fluorescence signal at 425 nm of 2-hydroxyterephthalic acid was in proportion to the amount of hydroxyl radicals produced in water. Fluorescence spectra of the generated 2-hydroxyterephthalic acid were measured on a Cary Eclipse fluorescence spectrophotometer (VARIAN Co, USA).3. Results and discussion
3.1. Characteristics of CdFe2O4
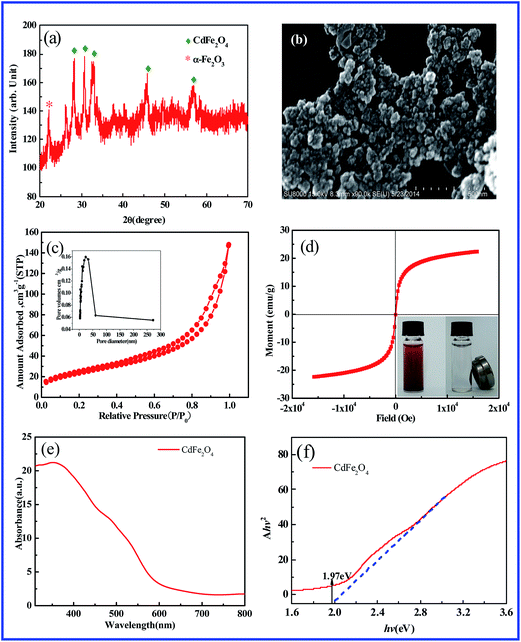
Fig. 1a shows the XRD pattern of as-synthesized CdFe2O4 powders. It is observed that the diffraction peaks could be well indexed to the spinel-type of CdFe2O4 (JCPDS 22-1063). The diffraction peak appeared below 25° indicated the presence of α-Fe2O3. Fig. 1b displays SEM micrograph of the obtained CdFe2O4, showing the spherical formation of the synthesized nanoparticles with diameter in the narrow size range of 20–30 nm. Some agglomerates of the CdFe2O4 particles are also observed because of the high surface energy and magnetic interactions between the nanocrystallites. The specific area of the sample was calculated using the Brunauer–Emmett–Teller (BET) method. Fig. 1c displays the N2 adsorption–desorption isotherm curve of the CdFe2O4. The BET surface area of CdFe2O4 was estimated to be 85.80 m2 g−1. The pore-size distribution of the samples was calculated by Barreet–Juyner–Halenda (BJH) method (see the inset of Fig. 1c). It can be seen that the average pore diameter of CdFe2O4 is 24 nm. The mesoporous structures would help the adsorption and transition of CR or its intermediates during the degradation process. Fig. 1d presents the hysteresis loops of the CdFe2O4 (inset: the photo of magnetic separation). The saturation magnetization of CdFe2O4 is 22.34 emu g−1. Since saturation magnetization of 16.3 emu g−1 was enough to separate magnetic particles from solution with a magnet,30 CdFe2O4 could be rapidly recovered from water. The UV-Vis diffuse reflectance spectrum of CdFe2O4 is illustrated in Fig. 1e. The plot obtained by the transformation based on the Kubelka–Munk function versus the energy of light is displayed in Fig. 1f. The estimated band gap value (Eg) was 1.97 eV for CdFe2O4.3.2. Degradation of CR by CdFe2O4 with MW irradiation
Fig. 2a shows the change of absorption spectra of CR in the presence of CdFe2O4. The absorption peak at λ = 497 nm diminished gradually with increasing irradiation time and completely disappeared after 10 min. No new absorption bands appeared in either the visible or the ultraviolet regions, which indicated the complete decolorization of CR during the reaction. | ||
| Fig. 2 (a) The variation of UV-Vis absorption spectra at different irradiation time; (b) removal efficiency of CR at different conditions; (c) the kinetic curves. | ||
The removal efficiency of CR over different conditions as a function of time was investigated (Fig. 2b). Self-degradation of CR was almost negligible without catalyst. And the removal efficiency of CR was approximately 26.5% after 10 min in CdFe2O4 dispersion without MW irradiation. Whereas CdFe2O4 displayed high catalytic performance, as almost 94.4% of CR was degraded under MW irradiation for only 10 min.
Fig. 2c shows the kinetic studies of the degradation of CR over different conditions. It was observed that the catalytic reaction obeyed the pseudo-first-order model according to the Langmuir–Hinselwood model and may be expressed as:
| ln(C0/C) = kt | (1) |
3.3. Influences of several factors
 | ||
| Fig. 3 The effect of the initial concentration (a) and dosage of CdFe2O4 (b) on the degradation of CR. | ||
Fig. 3b shows the dependence of CR (20 mg L−1) degradation on the dosage of the CdFe2O4 catalyst. It was found that the degradation ratios increase with increased dosage of CdFe2O4. And when the dosage exceeded 0.05 g, further increasing the dosage had negligible effect on the degradation ratio of CR after 10 min MW irradiation. Therefore, 0.05 g CdFe2O4 was used in our experiments.
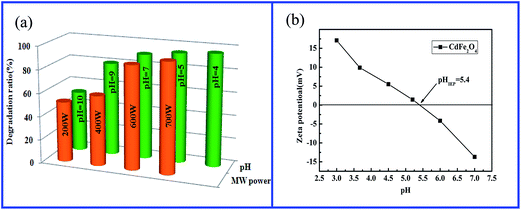 | ||
| Fig. 4 (a) The effect of microwave power and pH on the degradation of CR; (b) zeta potential of CdFe2O4. | ||
The influence of initial solution pH on CR degradation was also studied at catalyst dosage 0.05 g, CR concentration 20 mg L−1. Different experiments were performed at pH values of 4.0, 5.0, 7.0, 9.0, and 10.0 as shown in Fig. 4a. It was found that the degradation ratio of CR under MW irradiation changed scarcely and maintained a high level from pH 4.0 to pH 7.0, and then fell slightly after pH 9.0. In general, the natural pH of CR solution was close to 6.5. In this work, the CR solution without adjusting pH was popularly adopted.
The pH of the solution influenced the adsorption behavior of the organic dyes on the catalyst surface. When the pH value is lower than isoelectric point (pH 5.4, Fig. 4b) of CdFe2O4, its surface is positively charged. Whereas, when the pH value is higher than the isoelectric point, its surface is negatively charged. Therefore, in the acidic and neutral solution the CR anions are close to the surface of CdFe2O4 particles or adsorbed on it. Hence, the results exhibited a high degradation ratio of CR before pH 7.0. However, at higher pH values the CR anions were generally excluded away from the negatively charged surface of CdFe2O4. So the degradation ratio began to decrease.
Fig. 5b shows the effects of different anions (i.e. SO42−, NO3−, HCO3−, and CH3COO−) of their sodium salts at the same concentration of 0.001 mol L−1. Compared to the control test in the aqueous CR solutions without anion, NO3−, SO42−, and CH3COO− have no obvious influence on the CR degradation. And the existence of 0.001 mol L−1 HCO3− leads to the significant retardation of CR degradation, which can be attributed to the combination of radicals scavenging and competitive adsorption. HCO3− can be adsorbed by the catalyst. In addition, h+ and ˙OH can be trapped by HCO3− (eqn (2) and (3)).
| HCO3− + h+ → ˙HCO3− | (2) |
| HCO3− + ˙OH → ˙HCO3− + OH− | (3) |
3.4. Stability and reusability of CdFe2O4
The reusability of the catalyst is crucial in the practical application. To evaluate the catalytic stability of CdFe2O4, the particles were recovered using an external magnet to perform with successive tests of CR degradation. The morphologies of the CdFe2O4 after reaction were then examined. As shown in Fig. 6a, recycled CdFe2O4 showed strong activity in CR degradation and its activity remained almost unchanged in four cycles. The degradation ratios in all four runs are more than 90.0%. And the SEM image (Fig. 6b) shows that the sample still maintains the original morphology and particle size. These results strongly indicate that the CdFe2O4 nanoparticles are stable and recoverable as catalyst under MW irradiation. | ||
| Fig. 6 (a) Cyclic tests of the CdFe2O4 magnetic catalyst; (b) SEM image of the CdFe2O4 after the experiment of CR degradation. | ||
3.5. Identification of the intermediates and final products
In order to explore the intermediates in the degradation process, the chromatography of HPLC analysis was carried out at 497 nm and 345 nm, respectively. As shown in Fig. 7a, the matrix peak of CR appeared at tR = 4.28 min retention time, and it diminished gradually along with the increase of the irradiation time, suggesting CR was degraded obviously and rapidly. Synchronously, the peaks with tR = 4.28 min at 345 nm also significantly decreased (Fig. 7b). It could be seen that there was not any new peak appearing compared to the initial CR solution at both 497 nm and 345 nm, indicating no byproducts was identified. | ||
| Fig. 7 Variations of HPLC chromatograms of CR over CdFe2O4 with detection wavelength as 497 nm (a) and 345 nm (b); ion chromatogram of CR solution during degradation (c). | ||
For further validating degradation of CR in aqueous solution, the ionic chromatogram was determined as shown in Fig. 7c. The ionic chromatographic peaks corresponding to NO2−, NO3− and SO42− anions gradually increased along with microwave irradiation. All of these results indicated that the C–S, C–N and azo bonds in the CR molecule were destroyed gradually. The sulfur and nitrogen atoms were oxidized and transferred into NO2−, NO3− and SO42− anions, respectively. By all means, the CR in aqueous solution could be mineralized to a series of simple and innocuous inorganic ions in the end under microwave irradiation in the presence of CdFe2O4.
3.6. Role of the reactive species
To detect the main active oxidative species responsible for the degradation in the catalytic process, the influence of some radical scavengers on the degradation of CR over CdFe2O4 under MW irradiation was investigated. The effects of the scavengers on the degradation efficiency of CR are shown in Fig. 8a. It turned out that the catalytic degradation efficiency of CR decreased notably from 94.4% to 49.5% in the presence of 1 mM sodium oxalate (Na2C2O4, h+ scavenger32). Meanwhile, the rate of CR degradation (k) decreased obviously from 26.71 × 10−2 to 6.67 × 10−2 min−1, indicating that the holes are the main active species in the catalytic process. In addition, it has been observed that, upon addition of tert-butyl alcohol (TBA) (7.9 wt%), the rate of CR degradation decreases effectively (k = 21.54 × 10−2 min−1), which suggested that ˙OH is also responsible oxidants in degradation of CR. To test the role of ˙O2−, N2 or O2 was bubbled through the suspension. As shown in Fig. 8a, the negligible effect of N2 or O2 bubbling implies that ˙O2− is not the active species in this system.To further confirm the participation of ˙OH in degradation of CR, the fluorescence spectrophotometer was used to identify the generated ˙OH in the microwave-induced catalytic degradation process using terephthalic acid as a probe molecule. Terephthalic acid readily reacts with ˙OH to produce highly fluorescent 2-hydroxyterephthalic acid (Ex: 315 nm, Em: 425 nm). Fig. 8b shows the changes of fluorescence spectra from 3 × 10−4 M terephthalic acid solution with irradiation time. A gradual increase in fluorescence intensity at about 425 nm was observed, which further confirmed the production of ˙OH radicals.
In order to make clear the role of ˙O2− and ˙OH, we also used an electrochemical method to measure the flat-band positions (Vfb) of CdFe2O4. As seen from Fig. 8c, the Vfb value is roughly 0.18 V versus Ag/AgCl (equivalent to 0.38 V vs. NHE). Therefore, it can be determined that the conduction band potential (ECB) of CdFe2O4 is about 0.38 V. These accumulated electrons on the CB of CdFe2O4 cannot reduce O2 to yield ˙O2−, because the CB edge potential of CdFe2O4 (0.38 V vs. NHE) was more positive than the standard redox potential Eθ(O2/˙O2−) (−0.28 V vs. NHE).33 This explains why N2 or O2 bubbling has a negligible effect on the catalytic performance. According to the band gap energy (Eg = 1.97 eV, Fig. 1f) obtained from DRS measurement, the value band potential (EVB) of CdFe2O4 is deduced to be about 2.35 V vs. NHE, which is more positive than Eθ(˙OH/H2O) (2.27V vs. NHE).33 Therefore, the MW-generated holes left behind in the VB of CdFe2O4 can theoretically oxidize the hydroxyl group or H2O to produce ˙OH radicals.
3.7. Possible degradation methism
On the basis of the experimental results mentioned above, a possible catalytic process for the degradation of CR in MW/CdFe2O4 system could be proposed, as illustrated in Scheme 1. CdFe2O4 as microwave absorbent can strongly absorb and transfer microwave energy. Under the microwave irradiation, CdFe2O4 particle surface can produce great amount of “hot spots”. Meanwhile, the electrons in CdFe2O4 could oscillate under the microwave excitation. The “hot-spots” that MW generated could speed up the movement of electrons in CdFe2O4 to excite electrons and therefore generate electron/hole pairs. The leftover holes directly reacted with CR or interacted with the hydroxyl group or H2O to produce the ˙OH, which was an extremely strong oxidant for the mineralization of CR. In addition, the holes can also directly oxidize CR to harmless products. Thus, these evidences lead to the conclusion that the holes (h+) and ˙OH should be the main factor that responsible for CR oxidation in this system.4 Conclusion
In this study, CdFe2O4 has been synthesized by a hydrothermal process and characterized by various techniques such as SEM, XRD, BET VSM and DRS. The as-prepared CdFe2O4 exhibited excellent catalytic activity in the microwave-induced catalytic degradation process and magnetic property for easy separation using an external magnetic field. The degradation percentage of CR could reach up to 94.4% for 10 min. A possible degradation mechanism is proposed based on the experimental results. This work can provide an effective technology for dye wastewater treatment.Acknowledgements
This project was supported by the National Nature Science Foundation of China (NSFC51178212), Liaoning Provincial Department of education innovation team projects (LT2012001), the Shenyang Science and Technology Plan (F12-277-1-69), the Foundation of 211 project for Innovative Talent Training, Liaoning University and the Program for Liaoning representative office of China Environmental Protection Foundation (CEPF2013-123-1-5). The authors also thank their colleagues and other students who participated in this work.References
- Y. H. Hsien, C. F. Chang, Y. H. Chen and S. Cheng, Appl. Catal., B, 2001, 31, 241–249 CrossRef CAS.
- U. Zissi and G. Lyberatos, Water Sci. Technol., 1996, 34, 495–500 CrossRef CAS.
- Z. Abbasi, M. H. Shariat and S. Javadpour, Powder Technol., 2013, 249, 181–185 CrossRef CAS PubMed.
- A. Shavandi, A. E. D. A. Bekhit, A. Ali, Z. Sun and J. T. Ratnayake, Powder Technol., 2015, 273, 33–39 CrossRef CAS PubMed.
- B. Maté, R. D. Suenram and C. Lugez, J. Chem. Phys., 2000, 113, 192–199 CrossRef PubMed.
- S. Horikoshi, H. Hidaka and N. Serpone, Environ. Sci. Technol., 2002, 36, 1357–1366 CrossRef CAS.
- S. Horikoshi, A. Matsubara, S. Takayama, M. Sato, F. Sakai, M. Kajitani, M. Abe and N. Serpone, Appl. Catal., B, 2009, 91, 362–367 CrossRef CAS PubMed.
- T. L. Lai, J. Y. Liu, K. F. Yong, Y. Y. Shu and C. B. Wang, J. Hazard. Mater., 2008, 157, 496–502 CrossRef CAS PubMed.
- Z. Zhang, Y. Shan, J. Wang, H. Ling, S. Zang, W. Gao, Z. Zhao and H. Zhang, J. Hazard. Mater., 2007, 147, 325–333 CrossRef CAS PubMed.
- X. Zhang, Y. Wang, G. Li and J. Qu, J. Hazard. Mater., 2006, 134, 183–189 CrossRef CAS PubMed.
- A. K. L. Sajjad, S. Shamaila, B. Tian, F. Chen and J. Zhang, J. Hazard. Mater., 2010, 177, 781–791 CrossRef CAS PubMed.
- F. Han, V. S. R. Kambala, M. Srinivasan, D. Rajarathnam and R. Naidu, Appl. Catal., A, 2009, 359, 25–40 CrossRef CAS PubMed.
- X. Quan, X. Liu, L. Bo, S. Chen, Y. Zhao and X. Cui, Water Res., 2004, 38, 4484–4490 CrossRef CAS PubMed.
- J. E. Atwater and J. R. R. Wheeler, Appl. Phys. A, 2004, 79, 125–129 CrossRef CAS PubMed.
- T. L. Lai, C. C. Lee, K. S. Wu, Y. Y. Shu and C. B. Wang, Appl. Catal., B, 2006, 68, 147–153 CrossRef CAS PubMed.
- L. Zhang, X. Liu, X. Guo, M. Su, T. Xu and X. Song, Chem. Eng. J., 2011, 173, 737–742 CrossRef CAS PubMed.
- Y. Wu, P. Qiao, J. Qiu, T. Chong and T. S. Low, Nano Lett., 2001, 2, 161–164 CrossRef.
- H. Lin, H. Zhu, H. Guo and L. Yu, Mater. Lett., 2007, 61, 3547–3550 CrossRef CAS PubMed.
- L. Olmedo, P. Hourquebie and F. Jousse, Adv. Mater., 1993, 5, 373–377 CrossRef CAS PubMed.
- V. M. Petrov and V. V. Gagulin, Inorg. Mater., 2001, 37, 93–98 CrossRef CAS.
- X. Li, Y. Hou, Q. Zhao and L. Wang, J. Colloid Interface Sci., 2011, 358, 102–108 CrossRef CAS PubMed.
- Y. Yao, Y. Cai, F. Lu, F. Wei, X. Wang and S. Wang, J. Hazard. Mater., 2014, 270, 61–70 CrossRef CAS PubMed.
- J. Deng, Y. Shao, N. Gao, C. Tan, S. Zhou and X. Hu, J. Hazard. Mater., 2013, 262, 836–844 CrossRef CAS PubMed.
- S. Q. Liu, L. R. Feng, N. Xu, Z. G. Chen and X. M. Wang, Chem. Eng. J., 2012, 203, 432–439 CrossRef CAS PubMed.
- H. Chen, S. Yang, J. Chang, K. Yu, D. Li, C. Sun and A. Li, Chemosphere, 2012, 89, 185–189 CrossRef CAS PubMed.
- L. Zhang, X. Zhou, X. Guo, X. Song and X. Liu, J. Mol. Catal. A: Chem., 2011, 335, 31–37 CrossRef CAS PubMed.
- V. Vasanthi, A. Shanmugavani, C. Sanjeeviraja and R. Kalai Selvan, J. Magn. Magn. Mater., 2012, 324, 2100–2107 CrossRef CAS PubMed.
- F. Miao, Z. Deng, X. Lv, G. Gu, S. Wan, X. Fang, Q. Zhang and S. Yin, Solid State Commun., 2010, 150, 2036–2039 CrossRef CAS PubMed.
- R. M. Mohamed and E. S. Baeissa, Appl. Catal., A, 2013, 464–465, 218–224 CrossRef CAS PubMed.
- Z. Ma, Y. Guan and H. Liu, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 3433–3439 CrossRef CAS PubMed.
- H. C. Liang, X. Z. Li, Y. H. Yang and K.-H. Sze, Chemosphere, 2008, 73, 805–812 CrossRef CAS PubMed.
- B. Jiang, P. Zhang, Y. Zhang, L. Wu, H. Li, D. Zhang and G. Li, Nanoscale, 2012, 4, 455–460 RSC.
- A. Fujishima and X. Zhang, C. R. Chim., 2006, 9, 750–760 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |