Polyol synthesis of non-stoichiometric Mn–Zn ferrite nanocrystals: structural /microstructural characterization and catalytic application†
Abstract
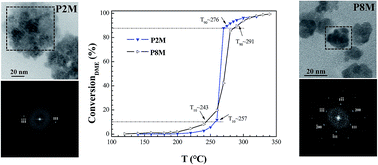
The structure of polyol-made Mn–Zn ferrite powders was investigated by X-ray diffraction, X-ray photoelectron spectrometry and X-ray absorption spectroscopy. It was found to be a defect-spinel structure in relation with non-stoichiometry introduced by the mixed oxidation-state of Mn and Fe cations. The ionic defects were identified as cation vacancies mainly located in the octahedral sites. The microstructure of these ferrites was studied by X-ray-diffraction and transmission electron microscopy. They appear to be constituted by quasi-isotropic monodisperse nano-aggregates which consist of pseudo-single crystals. Considering their reduced size, high crystalline quality, and cation mixed valence state related to their non-stoichiometry, the produced particles appear to be particularly valuable for redox-based solid–gas catalytic reactions. They exhibit an interesting activity toward catalytic combustion of dimethyl ether.

 Please wait while we load your content...
Please wait while we load your content...