MWCNT/perylene bisimide water dispersions for miniaturized temperature sensors†
Abstract
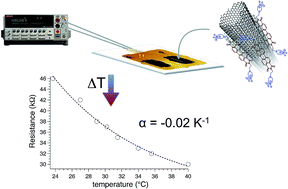
We report on a new ionic surfactant based on extended polycyclic aromatic perylene bisimides (PBI), namely N,N′-bis(2-(1-piperazino)ethyl)-3,4,9,10-perylenetetracarboxylic acid diimide dichloride (PZPERY), suitable for the exfoliation of multi-walled carbon nanotubes (MWCNTs). The ultrasonication (400 W for 5 min) of MWCNT/PZPERY water mixtures followed by centrifugation (4000 rpm for 30 min) provided disentangled and undamaged MWCNTs, which interact with the PZPERY molecules through π–π stacking interactions. The deposition of MWCNT/PZPERY dispersions on plastic film supporting gold electrodes allowed the fabrication of temperature sensors showing reproducible electrical resistances and typical semiconducting (activated) electrical transport with decreased resistivity when heated from 20 to 40 °C. The resistivity–temperature profile was very reproducible and with a negative temperature coefficient of about −0.02 K−1, a value comparable to that found in common thermistors. Our results suggest that MWCNT/PZPERY based films have utility for the formation of sensitive, stable and reproducible sensors to measure body temperature.

 Please wait while we load your content...
Please wait while we load your content...